Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Medication nonadherence is as high as 80% among SLE patients and leads to higher morbidity, mortality, and healthcare costs. Few studies have tested interventions to improve adherence in SLE patients, and none was effective among adults with SLE in the US. We developed an intervention based on prior study results and data from Surescripts, the largest health information network in the US, in collaboration with lupus clinic stakeholders, including patients, providers, and staff. The intervention uses Surescripts pharmacy refill data which draws from nearly all pharmacies and electronic health record systems, to monitor nonadherence and prompt tailored discussions surrounding medication use during the clinic encounter. We pilot-tested this intervention to assess feasibility, acceptability, appropriateness, and to explore its effect on adherence.
Methods: We conducted a 12-week pilot intervention at an university lupus clinic among consecutive follow-up patients on SLE medications, including HCQ, MTX, AZA, MMF, LEF, and belimumab. Adherence was assessed by medication possession ratio (MPR) based on Surescripts refill data, using MPR ≥80% as a cutoff for being adherent. During an encounter, the clinic note template prompted the provider to share the screen with the patient and review pharmacy refill data together (Figure 1). Patients with MPR ≥80% received encouraging statements. For those with MPR < 80%, providers asked open-ended questions to elicit and address adherence barriers. Discussions were documented in the note. We measured feasibility, acceptability, and appropriateness using patient and provider surveys. Feasibility was also assessed by medical record documentation. We explored change in adherence by comparing MPR in the 3-month periods before and after the intervention visit.
Results: All 6 lupus clinic rheumatologists participated, and 134 follow-up SLE patients were seen during the pilot period (mean age 43, 96% female, and 53% black). Provider surveys showed high scores for acceptability (4.4/5), appropriateness (4.6/5), and feasibility (4.7/5) of the intervention. Among 48 patients who completed surveys, the most common reactions to the intervention visit were feeling determined (24%), empowered (21%), and proud (12%). No patient reported being angry, upset, or embarrassed. Ninety percent of clinic notes contained documentation of reviewing Surescripts data with the patient. Adherence rates comparing 3 months before and after the intervention visit increased for HCQ (n=121) from 60% to 71% (p=0.02), for DMARDs (n=85) from 49% to 61% (p=0.06), and for MMF (n=37) from 50% to 59% (p=0.1).
Conclusion: We developed an intervention based on formative results in collaboration with lupus clinic stakeholders that showed excellent acceptability, appropriateness, and feasibility among patients and providers. The intervention led to a statistically significant improvement in HCQ adherence and a trend for improvement in DMARD and MMF adherence. Future work should elucidate the mechanisms by which this intervention works, assess its efficacy in a controlled setting, and adapt its use among other chronic rheumatic conditions.
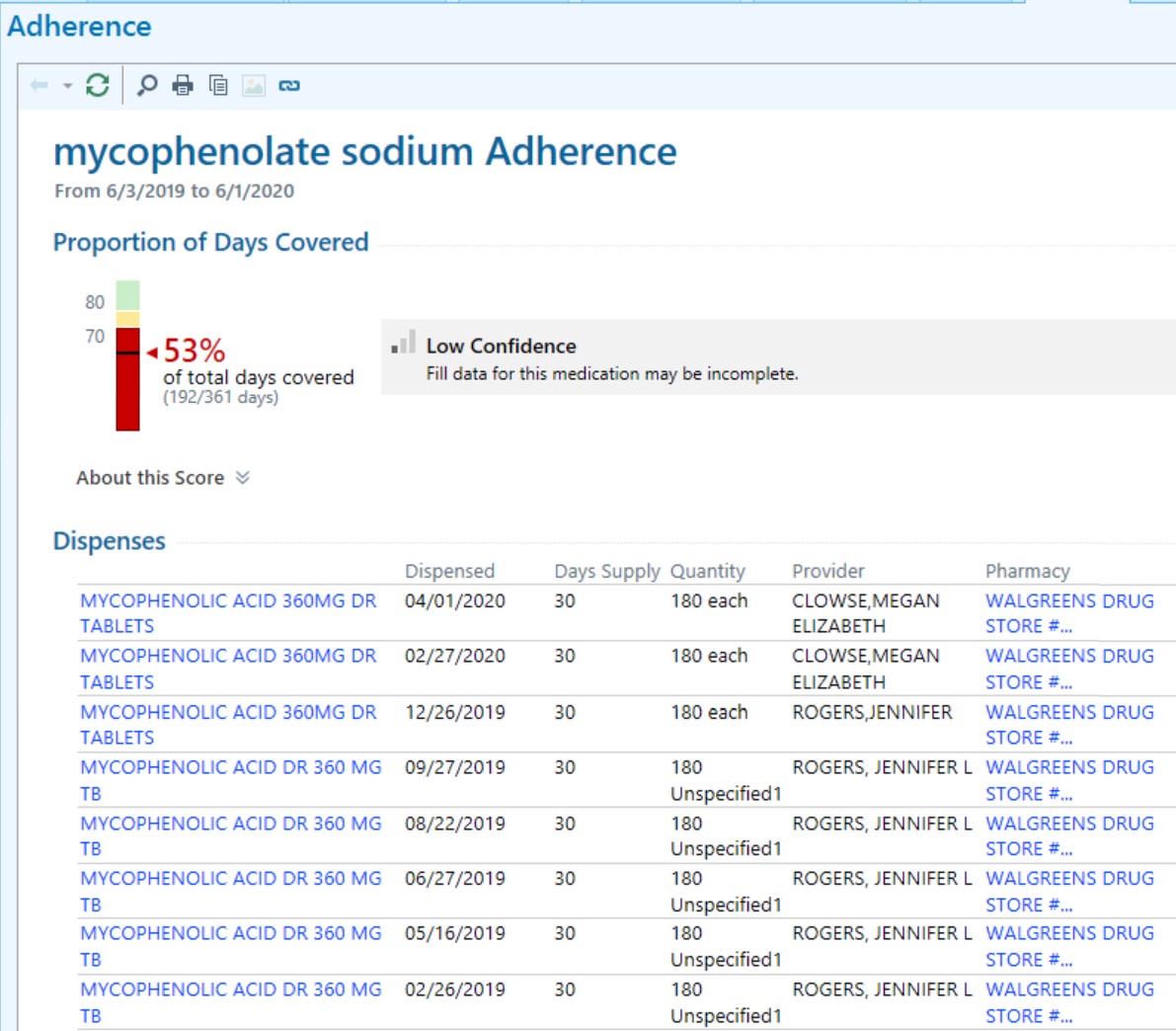 Figure 1. Screen shot of Surescripts pharmacy refill information providers review with patients during the clinical encounter.
Figure 1. Screen shot of Surescripts pharmacy refill information providers review with patients during the clinical encounter.
To cite this abstract in AMA style:
Sun K, Rogers J, Sadun R, Eudy A, Doss J, Criscione-Schreiber L, Barr A, Eder L, Maheswaranathan M, Corneli A, Bosworth H, Clowse M. Intervention to Improve SLE Medication Adherence Using Surescripts Pharmacy Refill Data [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/intervention-to-improve-sle-medication-adherence-using-surescripts-pharmacy-refill-data/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/intervention-to-improve-sle-medication-adherence-using-surescripts-pharmacy-refill-data/
