Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) affects around 50% of patients with systemic sclerosis (SSc) and is the main cause of mortality. Preliminary data from small cohorts suggest ILD may occur even in very early SSc (veSSc, pre-scleroderma). The main aim of this study is to assess the prevalence and the characteristics of ILD in veSSc and to identify possible predictors of ILD development during follow-up.
Methods: This is a longitudinal cohort study of a single EUSTAR center. We included patients with veSSc, defined as Raynaud’s phenomenon and/or at least one of puffy fingers, antinuclear antibodies (ANA), abnormal capillaroscopy, not fulfilling the 2013 ACR/EULAR classification criteria for SSc at baseline. ILD was diagnosed by expert radiologists on high-resolution computer tomography of the chest, which was performed yearly. We analyzed the clinical and spirometry parameters of ILD cases at baseline and during follow-up. Standardized mean difference (SMD) was used to compare patient groups with and without ILD. Cox regression analysis was used to identify risk factors for development of ILD in veSSc. Covariates were chosen according to previous literature and expert opinion.1
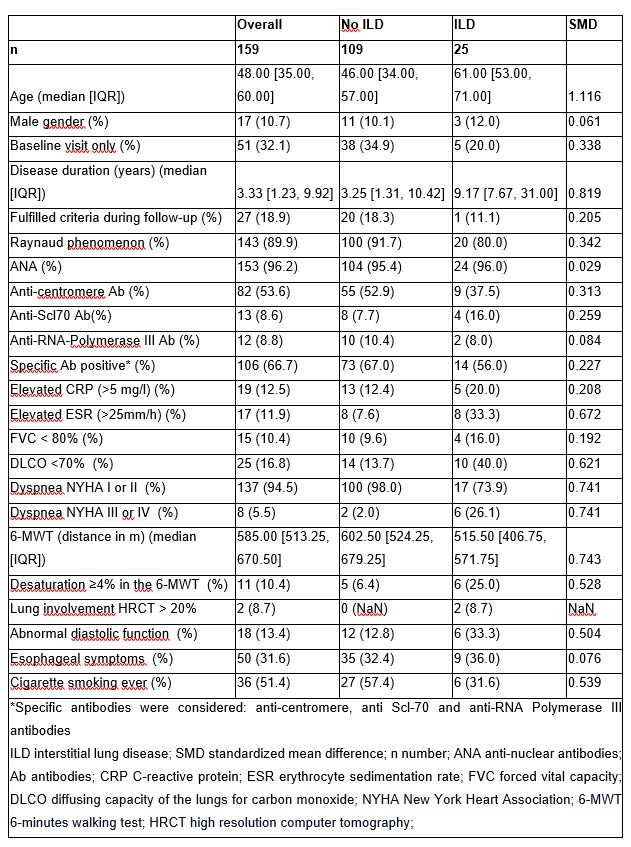
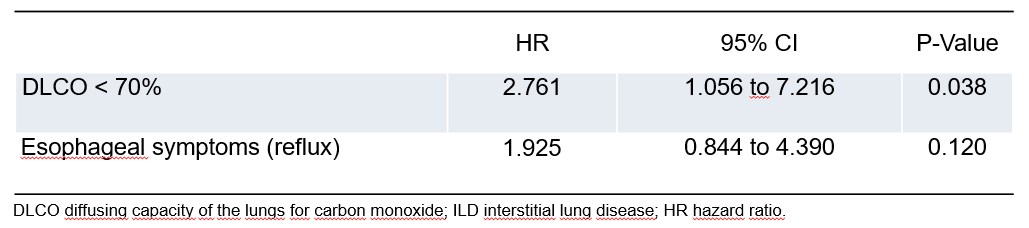
Results: In our cohort of 737 patients, we identified 159 patients with veSSc. Among these, 25 (15.7%) patients had ILD (Table 1). There were 9 ILD cases (5.7%) at baseline and 16 cases were first detected at follow-up. All of these patients fulfilled the classification criteria during the follow-up period, 10/16 at the visit when ILD was diagnosed. The groups with- and without ILD were imbalanced as measured by SMD: patients with ILD were older, had a longer disease duration (measured since the first occurrence of Raynaud phenomenon) and more often elevated inflammatory markers, especially ESR (Table 1). There was a significant association between ILD at baseline and a reduced diffusing capacity for carbon monoxide (defined as DLCO < 70% of predicted) (p=0.007, Fisher’s test). However, we neither found an association between ILD at baseline and a low forced vital capacity (FVC< 80% of predicted), nor with specific antibodies (anti-centromere, anti-Scl70 or anti-RNA polymerase III), presence of puffy fingers or a short disease duration. Only a DLCO< 70% at baseline was a predictor for developing ILD during the follow-up period (HR 2.761, 95% CI 1.056 to 7.216, p=0.038), whereas the presence of reflux symptoms was not significant in the regression model with these two covariates (Figure 1).
Conclusion: After a first analysis of ILD in veSSc, we observed that ILD occurs even in these very mild cases. Therefore, screening for ILD is important even in veSSc. A reduced DLCO < 70% at baseline was not only associated with ILD at baseline, but also predicted the development of ILD at follow-up.
References
1. Hoffmann-Vold AM et all Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis. 2021 Feb;80(2):219-227.
To cite this abstract in AMA style:
Muraru S, Mihai C, Hoffmann-Vold A, Bruni C, Elhai M, Becker M, Jordan S, Garaiman A, Distler O, Dobrota R. Interstitial Lung Disease in Very Early Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/interstitial-lung-disease-in-very-early-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interstitial-lung-disease-in-very-early-systemic-sclerosis/