Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Omics
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
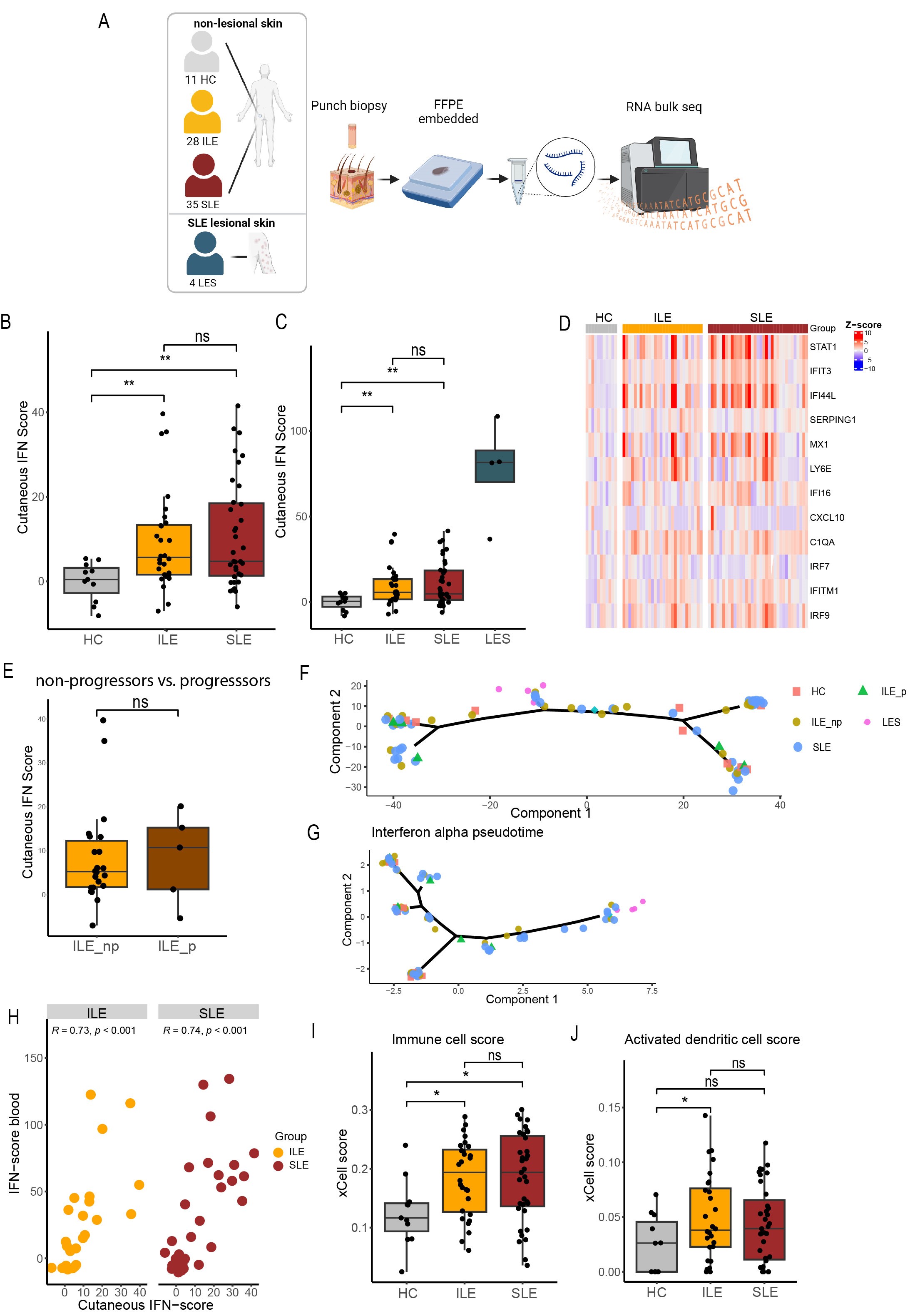
Background/Purpose: Patients with features of systemic lupus erythematosus (SLE) who do not fulfill classification criteria are referred to as incomplete SLE (ILE). These patients are at increased risk of progression to SLE. Cutaneous involvement is common in both ILE and SLE and lupus rashes often precede systemic flares. Recent data showed that interferons (IFN) are highly expressed in healthy-appearing, non-lesional skin of SLE patients, indicating that the skin is an immune-activated site in SLE. This might play an important role in induction of systemic symptoms and progression from ILE to SLE. Therefore, the aim of this study was to compare the IFN signature in non-lesional skin between healthy controls (HC), ILE and SLE patients.
Methods: In this cross-sectional study, we performed bulk RNA sequencing on biopsies obtained from non-lesional, non-sun-exposed skin from 11 HC, 28 ILE, 35 SLE patients and on 4 cutaneous lupus lesional biopsies. All ILE patients had an ANA titer of ≥ 1:80 and at least one additional SLICC criterium but not enough for classification. SLE patients all met the SLICC criteria. Selected interferon stimulated genes (ISGs) were summarized as IFN scores (Z-scores) and also measured in paired blood samples by qPCR. We performed pseudo-time trajectory analysis for the whole genome and ISGs to examine the differences in skin gene-expression at different stages of the pathogenesis of SLE. Furthermore, ILE patients were stratified into progressors and non-progressors, based on SLE classification up to 8 years after sample collection. Finally, for non-lesional skin, cell-type enrichment analysis was performed with xCell.
Results: ISGs were elevated to the same extent both in non-lesional skin of ILE and SLE patients compared to HC. Furthermore, non-lesional skin IFN scores correlated with IFN scores in blood. ISGs did not differ in non-lesional skin between ILE progressors and non-progressors. Pseudotime trajectory performed with non-lesional skin RNA sequencing data revealed no evident separation of HC, ILE and SLE patients. When performing pseudotime analysis with ISGs only, lesional samples showed clear separation from HC while ILE and SLE patients overlapped. In addition, cell-type enrichment analysis showed higher immune cell and higher activated dendritic cell scores in ILE and SLE patients compared to HC.
Conclusion: Our results suggest that non-lesional, healthy-appearing skin from patients with ILE harbors immunological changes similar to SLE. Therefore, cutaneous immune activation might be involved in early stages of lupus pathogenesis.
Abbreviations: HC: healthy control; ILE: incomplete systemic lupus erythematosus; SLE: systemic lupus erythematosus; LES: cutaneous lupus lesional skin; ILE_np: incomplete systemic lupus erythematosus non-progressors; ILE_p: incomplete systemic lupus erythematosus progressor; IFN: interferon; ISG: interferon stimulated genes; ns: not significant; * <0.05; ** <0.01¬.
Image A was created in BioRender.com.
To cite this abstract in AMA style:
Henning S, Tsoi L, Klein B, Berthier C, Kirma J, Wasikowski R, Diercks G, Horvath B, Bootsma H, Gudjonsson J, Westra J, de Leeuw K, Kahlenberg J. Interferon-stimulated Gene Expression in Non-lesional Skin Is Similarly Elevated in Incomplete SLE and SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/interferon-stimulated-gene-expression-in-non-lesional-skin-is-similarly-elevated-in-incomplete-sle-and-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/interferon-stimulated-gene-expression-in-non-lesional-skin-is-similarly-elevated-in-incomplete-sle-and-sle/