Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: The preferential Janus kinase (JAK)-1 inhibitor filgotinib (FIL) significantly improved signs and symptoms of RA in Phase 2 and 3 trials,1–5 and FIL is approved for treatment of moderately to severely active RA in Europe and Japan. Integrated safety analysis of FIL with patient data through 2019 was presented at the 2020 ACR virtual meeting.6 This abstract reports updated, as-treated data with increased study-drug exposure (median, 2.2 years [y]; maximum, 6.8 y).
Methods: Data were integrated from 2 Phase 2 (NCT01668641, NCT01894516), 3 Phase 3 (NCT02889796, NCT02873936, NCT02886728), and 2 long-term extension (LTE) (NCT02065700, NCT03025308) trials. Phase 2 and 3 LTE data were through Nov 2020 and Jun 2020, respectively. The as-treated analysis set included all available data for patients receiving ≥1 dose FIL 200 (FIL200) or 100 mg (FIL100), including those rerandomized to filgotinib for LTE. Exposure-adjusted incidence rates (EAIR)/100 patient-years exposure (PYE) of treatment-emergent adverse events (TEAEs; onset after first dose and no later than 30 days after last dose or new drug first dose date −1 day) and TEAEs of special interest (AESIs)—including serious infections, opportunistic infections, herpes zoster, adjudicated major adverse cardiovascular events, adjudicated venous thromboembolism, atrial systemic thrombotic events, nonmelanoma skin cancer (NMSC), non-NMSC malignancies, and gastrointestinal perforations—are presented. EAIR and 95% CIs were estimated with Poisson models with treatment and study as covariates and natural log of exposure as offset, unless otherwise specified.
Results: 3691 patients received FIL200 or FIL100 for 8085.1 PYE (median 2.2, maximum 6.8 y). In the as-treated set, 61% of FIL200 and 45% of FIL100 patients received filgotinib for ≥2 years (Table 1). EAIR for TEAEs was higher with FIL100 than with FIL200; EAIRs for deaths were 0.5 and 0.3 for FIL200 and FIL100 (Table 2). Incidences of infections and serious infections were numerically greater for FIL100 vs FIL200, while EAIRs for other AESIs were comparable between doses (Table 3). EAIRs for AESIs tended to decrease since the previous update, except for VTE (total FIL 0.1 to 0.2) and non-NMSC malignancies (total FIL 0.5 to 0.6).
Conclusion: With 1 additional year of exposure since the 2020 report, FIL continues to be well tolerated with no new safety concerns emerging. EAIRs of TEAEs, including deaths, and AESIs remained stable or decreased since the 2020 report, except for slight increases in rates of NMSC and non-NMSC malignancies. In the context of demonstrated efficacy, both FIL doses had an acceptable risk/benefit profile.
References: 1. Westhovens. Ann Rheum Dis 2017;76:998–1008. 2. Kavanaugh. Ann Rheum Dis 2017;76:1009–1019. 3. Combe B et al. Ann Rheum Dis. Epub ahead of print: Jan 27, 2021. doi:10.1136/annrheumdis-2020-219214. 4. Genovese. JAMA 2019;322(4):315–325. 5. Westhovens R et al. Ann Rheum Dis. 2021;80:727–738. 6. Winthrop K et al. Arthritis Rheumatol. 2020;72(suppl 10);abstract 0229.
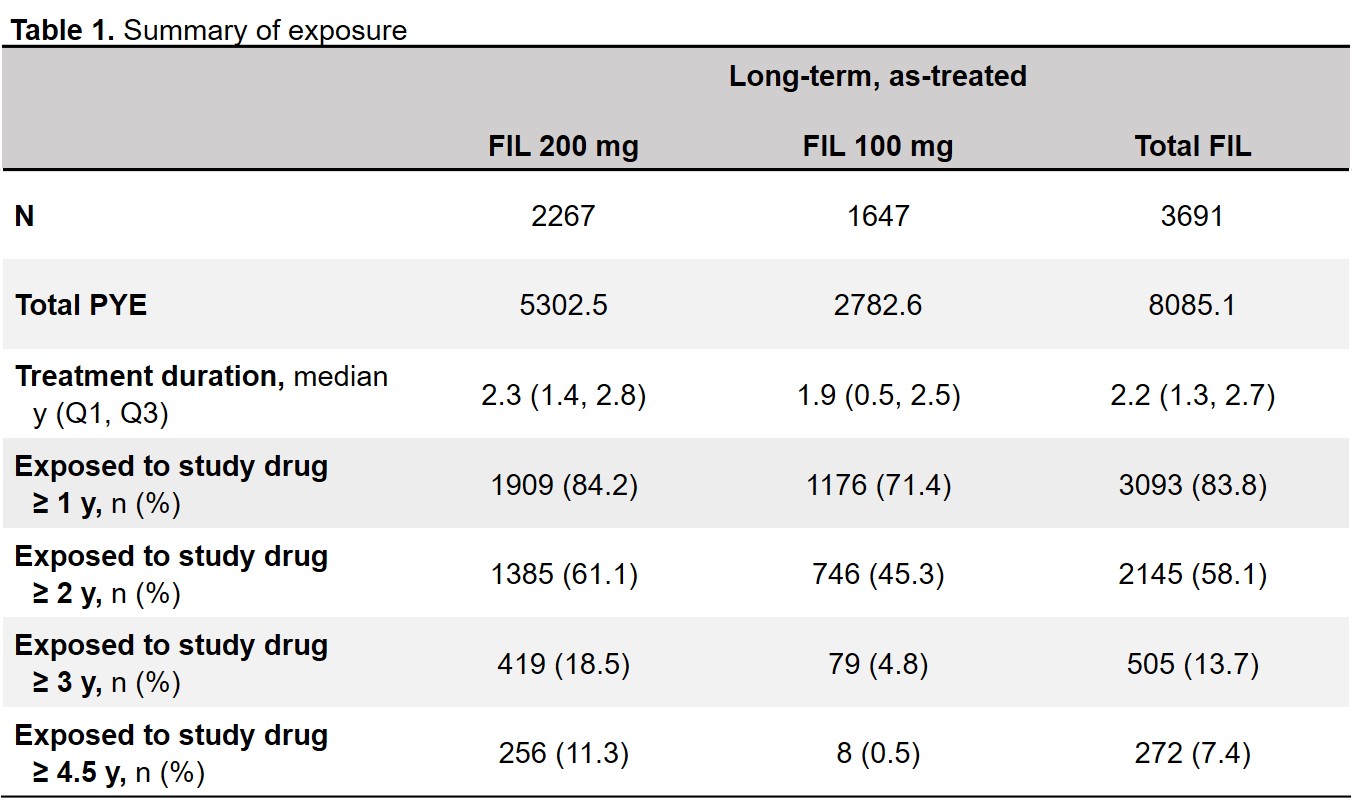 PYE was defined as (last dose date − first dose date + 1)/365.25. Duration of exposure to study drug (years) = (last dosing date − first dosing date + 1)/365.25. A 7-day window was applied to the on-treatment Week 52 to match with the protocol-specified visit window. FIL, filgotinib; PYE, patient-years of exposure; Q, quartile; y, years.
PYE was defined as (last dose date − first dose date + 1)/365.25. Duration of exposure to study drug (years) = (last dosing date − first dosing date + 1)/365.25. A 7-day window was applied to the on-treatment Week 52 to match with the protocol-specified visit window. FIL, filgotinib; PYE, patient-years of exposure; Q, quartile; y, years.
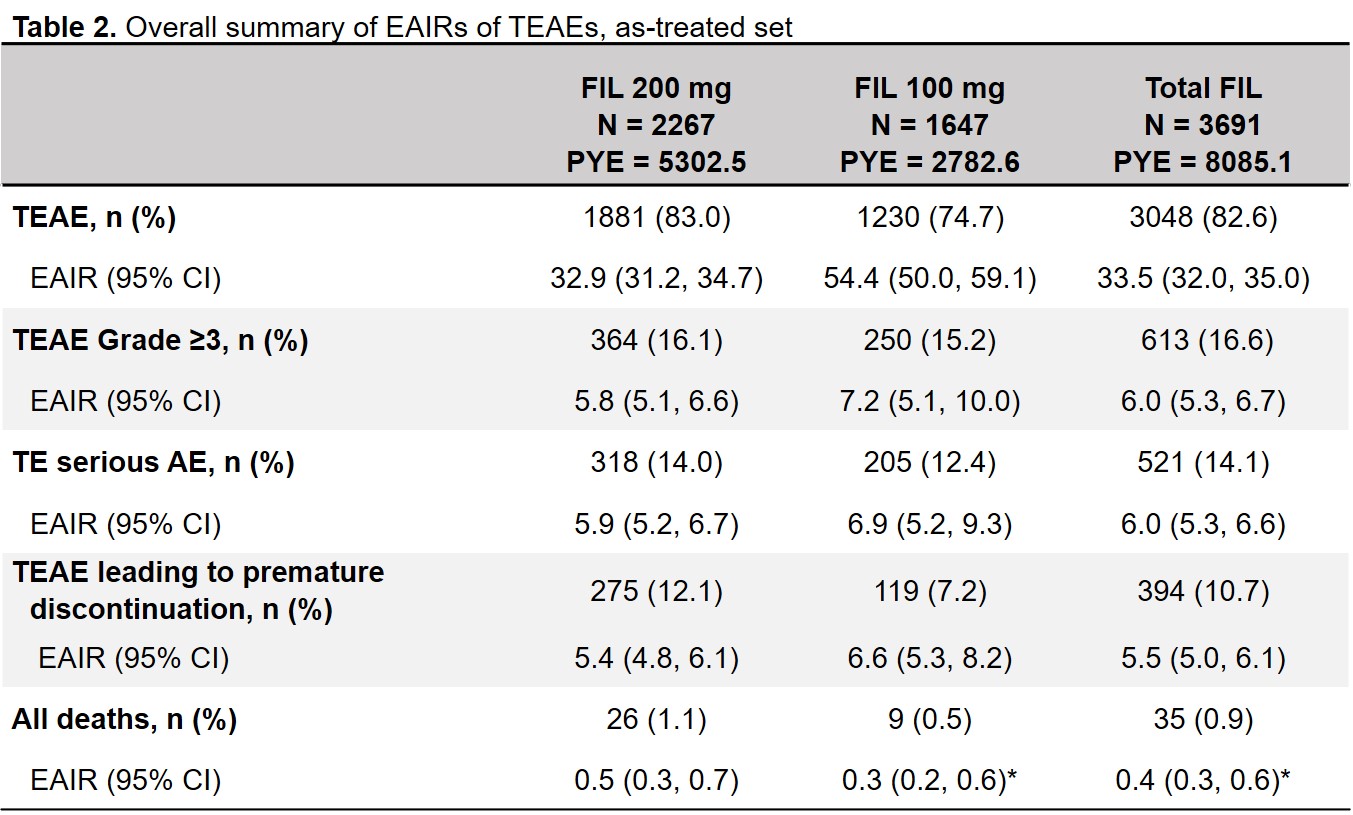 *Except when any study had 0 event within the treatment, the Poisson model was not adjusted by study. PYE was defined as (last dose date − first dose date + 1)/365.25. AE, adverse event; CI, confidence interval; EAIR, exposure-adjusted incidence rate per 100 PYE; FIL, filgotinib; PYE, patient-years of exposure; TE, treatment-emergent; TEAE, treatment-emergent adverse event.
*Except when any study had 0 event within the treatment, the Poisson model was not adjusted by study. PYE was defined as (last dose date − first dose date + 1)/365.25. AE, adverse event; CI, confidence interval; EAIR, exposure-adjusted incidence rate per 100 PYE; FIL, filgotinib; PYE, patient-years of exposure; TE, treatment-emergent; TEAE, treatment-emergent adverse event.
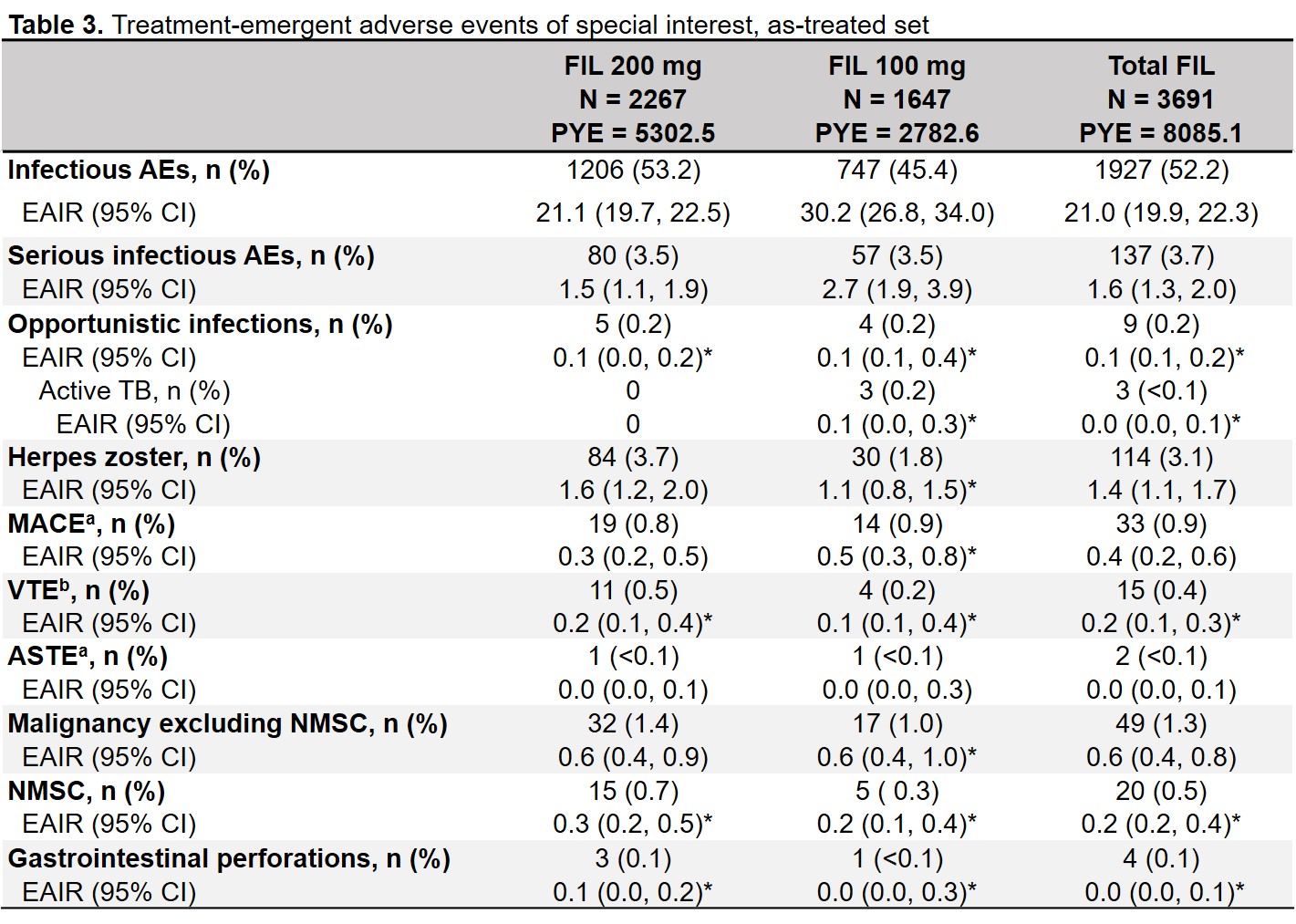 *Except when any study had 0 event within the treatment, the Poisson model was not adjusted by study. PYE was defined as (last dose date − first dose date + 1)/365.25. aPositively adjudicated. bAdjudicated as DVT or PE AE, adverse event; AESI, AE of special interest; ASTE, arterial systemic thrombotic event; CI, confidence interval; DVT, deep vein thrombosis; EAIR, exposure adjusted incidence rate per 100 PYE; FIL, filgotinib; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PE, pulmonary embolism; PYE, patient years of exposure; TB, tuberculosis; TE, treatment emergent; VTE, venous thromboembolism.
*Except when any study had 0 event within the treatment, the Poisson model was not adjusted by study. PYE was defined as (last dose date − first dose date + 1)/365.25. aPositively adjudicated. bAdjudicated as DVT or PE AE, adverse event; AESI, AE of special interest; ASTE, arterial systemic thrombotic event; CI, confidence interval; DVT, deep vein thrombosis; EAIR, exposure adjusted incidence rate per 100 PYE; FIL, filgotinib; MACE, major adverse cardiovascular events; NMSC, non-melanoma skin cancer; PE, pulmonary embolism; PYE, patient years of exposure; TB, tuberculosis; TE, treatment emergent; VTE, venous thromboembolism.
To cite this abstract in AMA style:
Winthrop K, Tanaka Y, Takeuchi T, Kivitz A, Genovese M, Pechonkina A, Matzkies F, Bartok B, Chen K, Jiang D, Tiamiyu I, Besuyen R, Strengholt S, Burmester G, Gottenberg J. Integrated Safety Analysis Update for Filgotinib in Patients with Moderately to Severely Active Rheumatoid Arthritis Receiving Treatment over a Median of 2.2 Years [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/integrated-safety-analysis-update-for-filgotinib-in-patients-with-moderately-to-severely-active-rheumatoid-arthritis-receiving-treatment-over-a-median-of-2-2-years/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/integrated-safety-analysis-update-for-filgotinib-in-patients-with-moderately-to-severely-active-rheumatoid-arthritis-receiving-treatment-over-a-median-of-2-2-years/
