Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate is the preferred initial drug for treatment of rheumatoid arthritis (RA) per American College of Rheumatology guidelines (2015). People with elderly-onset RA, classified as onset of disease after 60-65 years of age, are frequently undertreated with biologic disease modifying agents (bDMARDs) farther along the course of disease. However, little is known is about the initial management of RA in older patients. Using Medicare data, we examine the pattern in initial drug therapy in elderly onset RA patients.
Methods: We used Medicare claims from the years 2012 – 2016 to identify older adults ( >65 years) with RA defined on the basis of a single ICD9/ICD10 claim in hospital claims, two or more ICD9/ICD10 claims in outpatient claims (7 days apart but within 365 days), or single ICD9/ICD10 claim in outpatient claim plus a prescription or outpatient claim for a disease modifying agents (DMARDs). Study subjects were enrolled in Medicare fee-for-service for at least 12 months prior to case ascertainment. We excluded patients with any prior RA claim or DMARD claims in the prior 12 months to establish an incident RA cohort. Measures included baseline demographics and comorbidities, initial DMARDs used after diagnosis of RA along with their initial average daily dose prescribed and average steroids dose used in the first 90 days after first diagnosis.
Results: We identified 99,491 incident cases of RA (see Table 1 for baseline characteristics). The average time from first claim for RA to the first prescription of DMARD was 28.6 days. Conventional DMARDs (cDMARD) were used as an initial drug in 13.9% patients as compared to bDMARDs in 0.3% patients; 45.9% of patients received steroids within the first 3 months. Of all patients starting a cDMARD, hydroxychloroquine (41.04%) and methotrexate (35.72%) were the most common initial cDMARDs. Methotrexate initiation declined with increasing age (36.2% in 65-69-year age group v. 30.77% in >85-year age group). As the age of onset increased, doses of hydroxychloroquine, methotrexate, leflunomide and steroids decreased. Adalimumab and infliximab were the most prescribed bDMARDs.
Conclusion: In a national of elderly-onset RA patients, hydroxychloroquine instead of methotrexate was the preferred initial cDMARD, which could lead to inadequate control of disease. Further, with increasing age, the doses of cDMARDs prescribed reduced which could further contribute to reduced control of early disease.
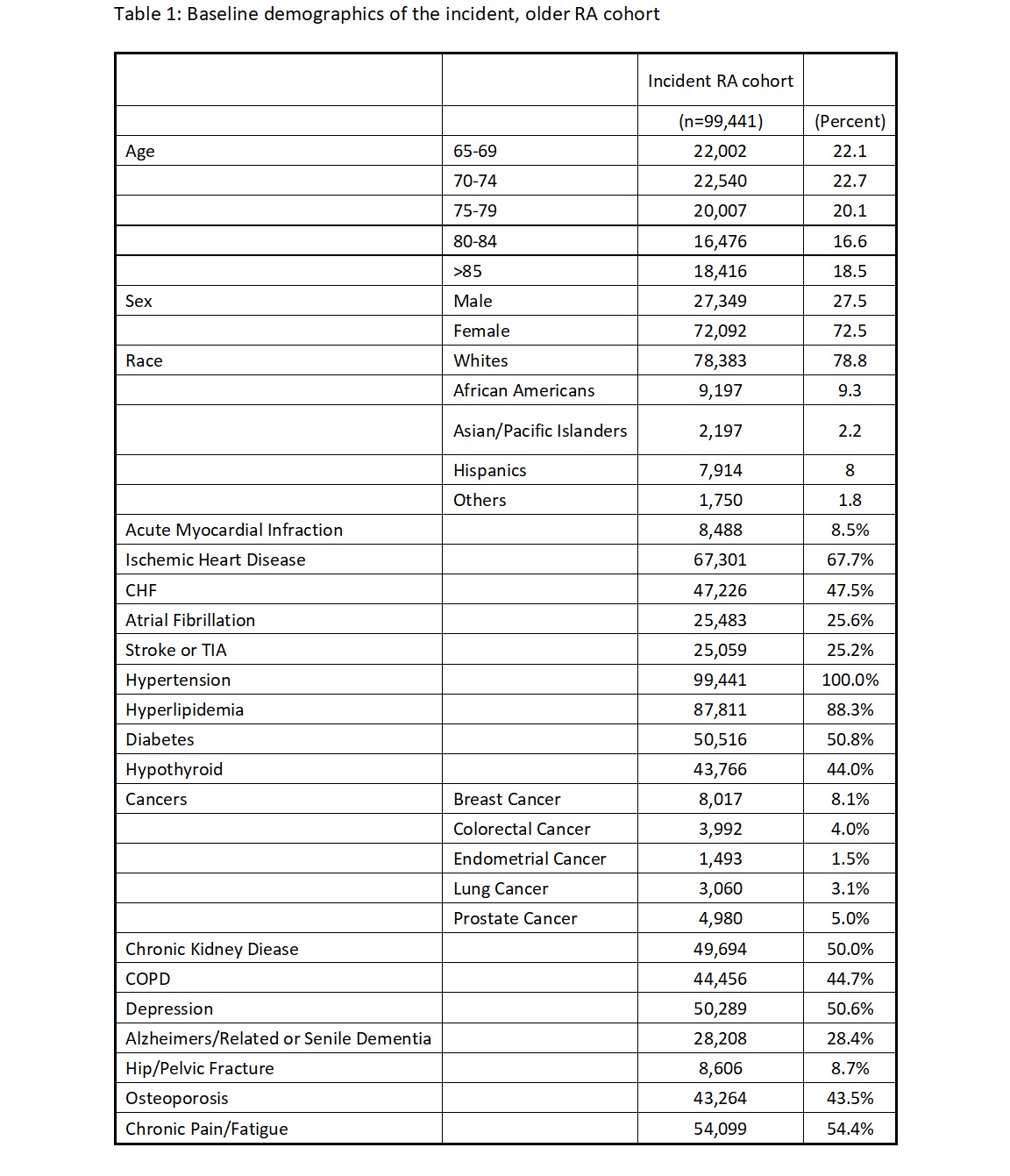 Table 1: Baseline demographics of the incident, older RA cohort
Table 1: Baseline demographics of the incident, older RA cohort
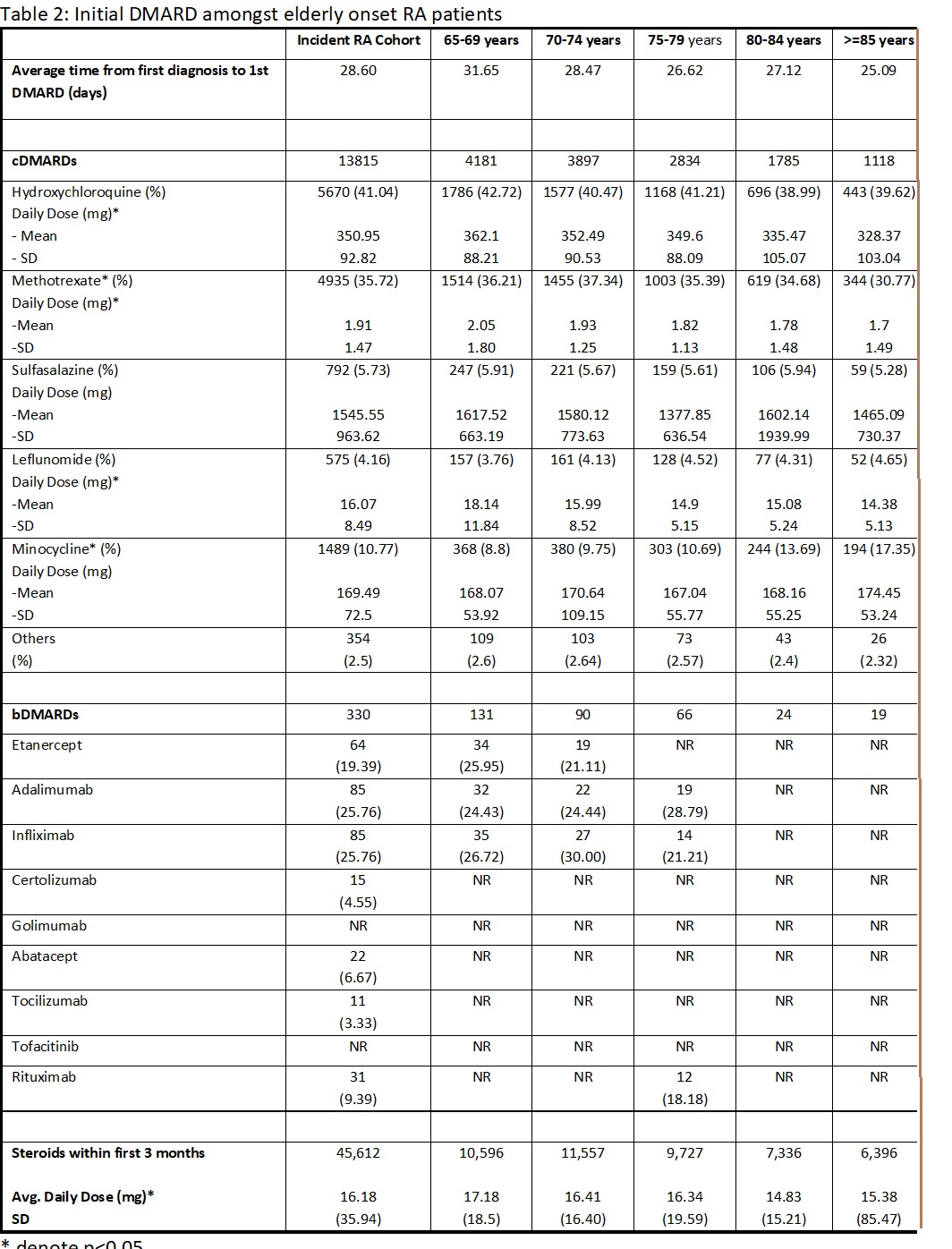 Table 2: Initial DMARD therapy amongst elderly onset RA patients
Table 2: Initial DMARD therapy amongst elderly onset RA patients
To cite this abstract in AMA style:
Dalal D, Zhang T, Varma H, Shireman T. Initial Pharmaceutical Management in a National Cohort of Elderly-Onset Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/initial-pharmaceutical-management-in-a-national-cohort-of-elderly-onset-rheumatoid-arthritis-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-pharmaceutical-management-in-a-national-cohort-of-elderly-onset-rheumatoid-arthritis-patients/
