Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitor-induced inflammatory arthritis (ICI-IA) occurs in 3-7% of patients receiving ICI therapy for cancer. ICI-IA is routinely treated with corticosteroids, however, there is no accepted standard regimen. There is concern that higher dose steroids may negatively affect tumor response to ICI therapy, while lower dose steroids may inadequately treat ICI-IA symptoms. This study investigated the effects of initial corticosteroid exposure on short- and long-term outcomes of disease activity, survival, and tumor progression.
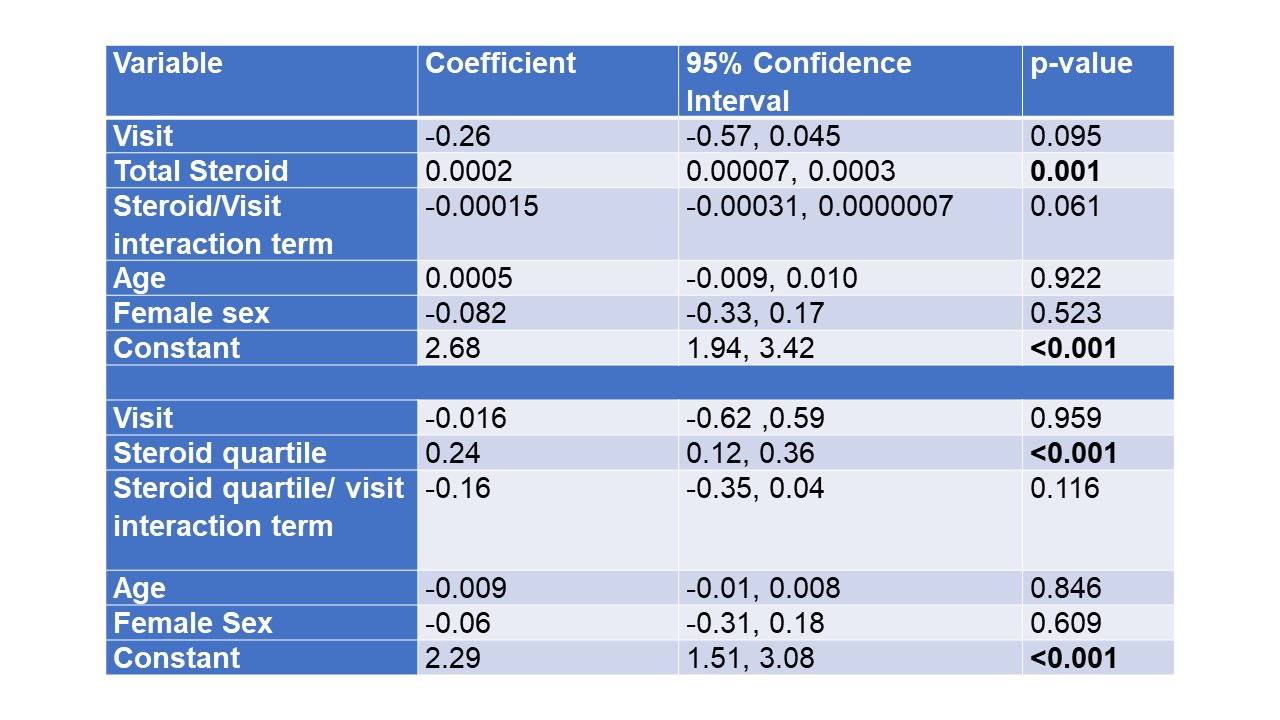
Methods: Patients with rheumatologist diagnosed ICI-IA and at least one follow up visit were included. Cumulative steroid exposure (prednisone equivalents) in the first 16 weeks after rheumatology baseline visit was determined from the prospective database as well as chart review. Patients were followed at subsequent visits to monitor survival and tumor progression. Total steroid dosing was analyzed in two ways; as a continuous variable and in four quartiles (1st quartile 0-349 mg, 2nd quartile 350-840 mg, 3rd quartile 841-1434 mg range, 4th quartile 1435-6507 mg). Clinical disease activity index (CDAI) scores were used to model disease activity over time with generalized estimating equations. Overall survival and time to tumor progression were evaluated with Kaplan-Meier curves and Cox proportional hazards models.
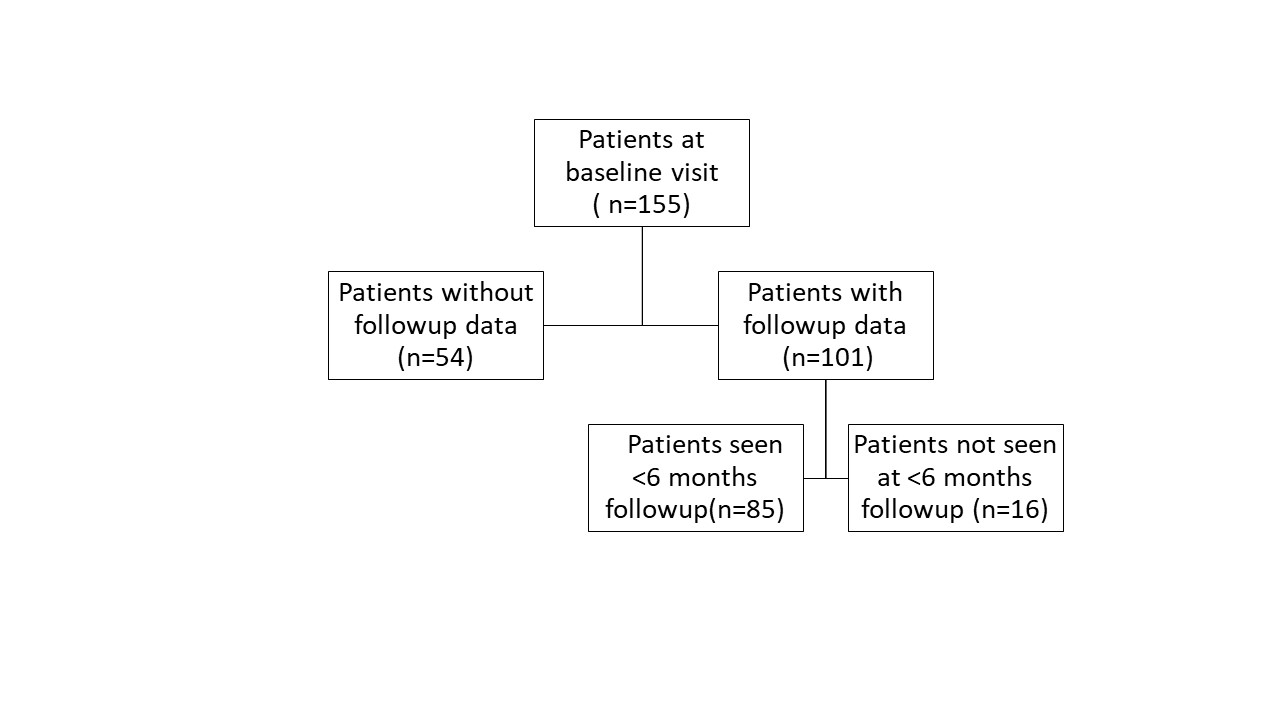
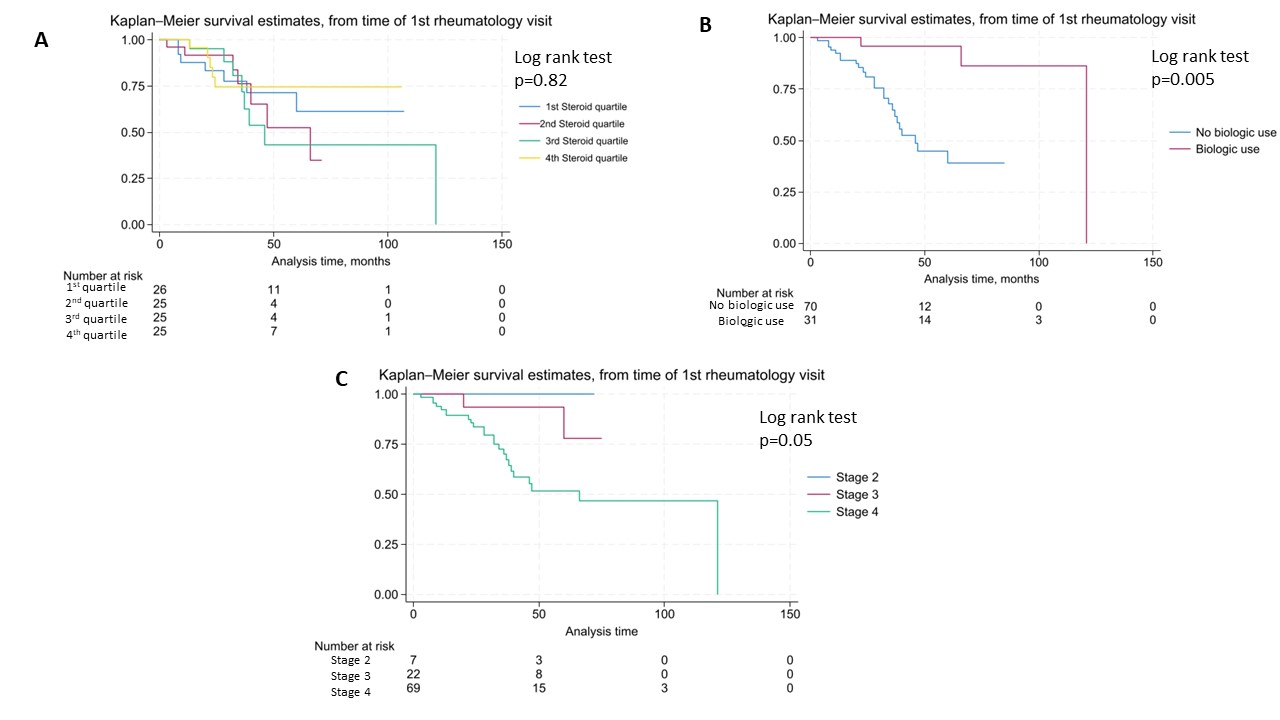
Results: Of 155 patients with ICI-IA, 101 patients had follow-up data available (mean duration 649 days) and 85 were seen within 6 months of baseline (figure 1). Mean age was 60 (SD 13) years, 54% were female, and 96% identified as White, 2% as Black and 3% as Asian. There was no significant decrease in CDAI in patients treated with higher doses of steroids when modeled by initial total steroid dose or steroid quartile (Steroid/visit interaction terms not significant, Table 1); those with higher initial CDAI did receive significantly higher doses of steroid. Overall survival was evaluated from time of rheumatology baseline visit. Kaplan-Meier curves showed no difference in survival by steroid quartile (figure 2A). On the other hand, there was decreased survival with higher cancer stage and increased survival in those treated with biologics for ICI-IA (figures 2B, C). Cox proportional hazards models showed no significantly increased hazard of death by total steroid dose (HR 1.0002, p=0.237); this was also true when controlling for cancer stage, time from ICI start, and ever biologic use (HR 1.0002, p=0.123). For tumor progression, there was a trend toward increase in hazard of progression based on total steroid dose (HR 1.0003, p=0.082), the trend persisted when controlling for time from ICI start, biologic use and cancer stage (HR 1.0002, p= 0.095).
Conclusion: There was no significant difference in improvement of CDAI scores at first follow up with higher steroid dosing. There was no significantly increased hazard of death for those treated with higher doses of steroids in follow up, but a trend toward increased hazard of tumor progression. Given the conflicting results for overall survival and tumor progression, further studies are needed to address survival bias and clarify the safety of steroids and steroid-sparing immunosuppression in ICI-IA.
To cite this abstract in AMA style:
Bukhari M, Shah A, Bingham C, Jones M, Cappelli L. Initial Corticosteroid Dosing and Effects on Disease Activity, Survival, and Tumor Progression in Immune Checkpoint Inhibitor-induced Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/initial-corticosteroid-dosing-and-effects-on-disease-activity-survival-and-tumor-progression-in-immune-checkpoint-inhibitor-induced-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-corticosteroid-dosing-and-effects-on-disease-activity-survival-and-tumor-progression-in-immune-checkpoint-inhibitor-induced-inflammatory-arthritis/