Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: This study aimed to evaluate the infection risks in patients with rheumatoid arthritis (RA) treated with biological or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), either as monotherapy or in combination with conventional synthetic DMARDs (csDMARDs).
Methods: A comprehensive literature search of MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) was conducted to identify randomized controlled trials (RCTs) assessing infection risks in RA patients treated with b/tsDMARDs. The primary outcome was the incidence of serious infections, while the secondary outcomes included any infection and specific events such as respiratory tract infections, gastroenteritis and herpes zoster. A frequentist network meta-analysis was performed to calculate odds ratios (ORs). This study was registered with PROSPERO (CRD42023435681).
Results: In this network meta-analysis of 127 RCTs involving 55,749 patients, b/tsDMARD monotherapy showed a similar risk of serious infections compared with csDMARDs. By contrast, combining csDMARDs with adalimumab, infliximab, tofacitinib, or upadacitinib (though not with other b/tsDMARDs) significantly increased the risk of serious infections (OR 1.51, 95% CI: 1.04-2.19; OR 1.75, 95% CI: 1.09-2.81; OR 2.52, 95% CI: 1.26-5.03; OR 2.31, 95% CI: 1.13-4.73, respectively). For any infection, b/tsDMARD monotherapy posed a similar risk to csDMARDs, except for etanercept. Regarding specific risks, tsDMARDs were associated with an increased incidence of herpes zoster compared to csDMARDs, except for filgotinib and peficitinib. Although rare, pneumonia, tuberculosis, and sepsis were observed more frequently with infliximab combined with csDMARDs than with other b/tsDMARD regimens.
Conclusion: Compared to csDMARDs, b/tsDMARD monotherapy showed no elevated serious infection risk, whereas specific combination therapy regimens with csDMARDs increased the risk of serious infections in RA patients. A specialized clinical pathway with detailed recommendations was developed to assess infection risks and optimize drug selection for RA patients undergoing b/tsDMARD treatment.
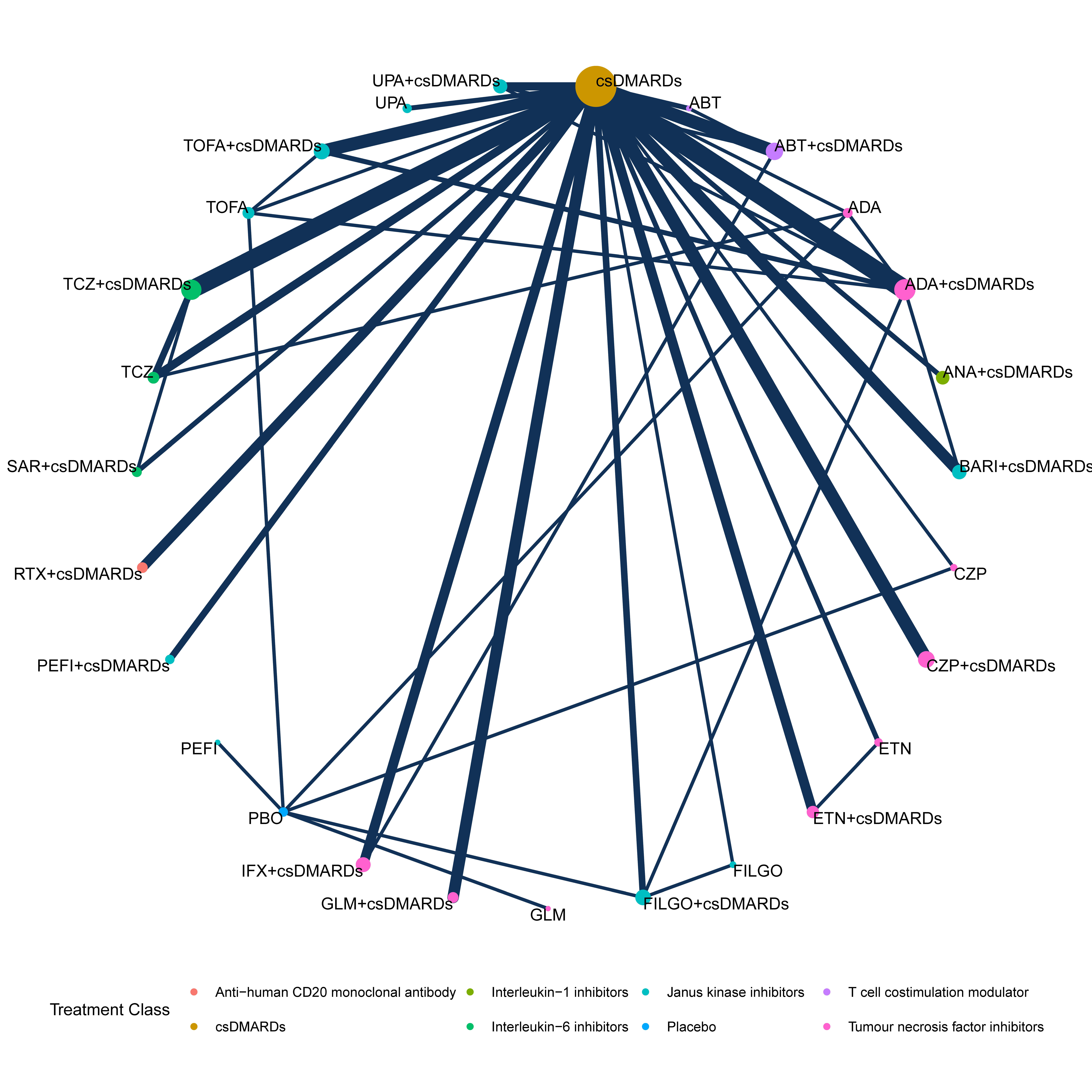 Figure 1 Network map of treatment comparisons for Serious infections
Figure 1 Network map of treatment comparisons for Serious infections
Links between the nodes indicated the available direct comparisons between different treatments. ABT, Abatacept; ADA, Adalimumab, ANA, Anakinra, BARI, Baricitinib; CZP, Certolizumab; ETN, Etanercept; FILGO, Filgotinib; GLM, Golimumab; IFX, Infliximab; PEFI, Peficitinib; PBO, Placebo; RTX, Rituximab, SAR, Sarilumab; TCZ, Tocilizumab; TOFA, Tofacitinib; UPA, Upadacitinib; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs.
.jpg) Table 1 A heat map ranking b/tsDMARDs ± csDMARDs based on the risk of serious infections, any infection, and specfic infectious outcomes
Table 1 A heat map ranking b/tsDMARDs ± csDMARDs based on the risk of serious infections, any infection, and specfic infectious outcomes
Numbers reflect P-score, which rank b/tsDMARDs ± csDMARDs on a continuous scale from 0 to 1. A higher P-score indicates a greater increase in risk of infection. Transverse line indicate that data were not available. ABT, Abatacept; ADA, Adalimumab, ANA, Anakinra, BARI, Baricitinib; CZP, Certolizumab; ETN, Etanercept; FILGO, Filgotinib; GLM, Golimumab; IFX, Infliximab; PEFI, Peficitinib; PBO, Placebo; RTX, Rituximab, SAR, Sarilumab; TCZ, Tocilizumab; TOFA, Tofacitinib; UPA, Upadacitinib, bDMARDs, biological disease-modifying antirheumatic drugs; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs.
.jpg) Figure 2. Infection Risk Management Protocol in Rheumatoid Arthritis
Figure 2. Infection Risk Management Protocol in Rheumatoid Arthritis
RA, rheumatoid arthritis; DMARDs, disease-modifying antirheumatic drugs; csDMARDs, conven-tional synthetic disease-modifying antirheumatic drugs; bDMARDs, biological disease-modifying antirheumatic drugs; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs; IL-1Ra, interleukin-1 receptor antagonist.
To cite this abstract in AMA style:
Liu L, Zhang X, Gu Z, Li J. Infection Risks Associated with Monotherapy and Combination Therapies Using Biological or Targeted – DMARD in RA: A Systematic Review and Network Meta-analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/infection-risks-associated-with-monotherapy-and-combination-therapies-using-biological-or-targeted-dmard-in-ra-a-systematic-review-and-network-meta-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infection-risks-associated-with-monotherapy-and-combination-therapies-using-biological-or-targeted-dmard-in-ra-a-systematic-review-and-network-meta-analysis/
