Session Information
Date: Monday, November 14, 2022
Title: RA – Treatment Poster IV
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Short-term (< 3 months) treatment with glucocorticoids (GC) (“bridging”) is recommended in the EULAR 2019 rheumatoid arthritis (RA) recommendations when starting a csDMARD in newly diagnosed patients. The updated ACR 2021 guidelines conditionally recommend not to start GC next to a csDMARD in these patients, because of concerns that patients are unable to discontinue GC, thereby risking serious toxicity. A previous review revealed that reported data on successful GC discontinuation after bridging are scarce. In this individual patient data (IPD) meta-analysis of clinical trials we evaluate how many patients used GC after initial “bridging” and if there are factors affecting this.
Methods: Data from 7 clinical trials using GC bridging (COBRA, BeSt, COBRA light, IMPROVED, tREACH, IDEA, CareRA), out of 10 identified trials in a previous systematic literature review, were available and combined in an IPD meta-analysis in newly diagnosed RA patients (they met the ACR 1987 classification criteria for RA in 5/7 studies, the 2010 EULAR/ACR classification criteria in 1/7 studies and 1 study used a clinical diagnosis of RA). Mixed-effects regression analyses with study arm as random effect were conducted. Main outcomes were GC status at several timepoints, cumulative GC dose and continuous GC use for ≥3 months after initial bridging. Initial GC dose, initial co-treatment, mode of GC administration (oral vs. non-oral) and intended duration of bridging were added to these models in univariable analyses to test their association with each outcome.
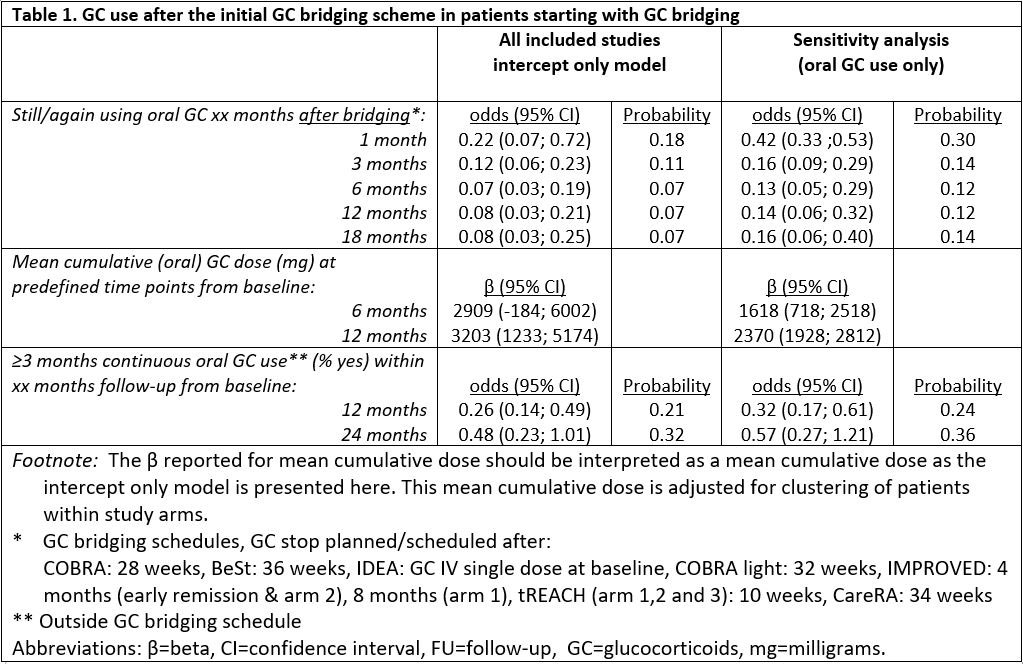
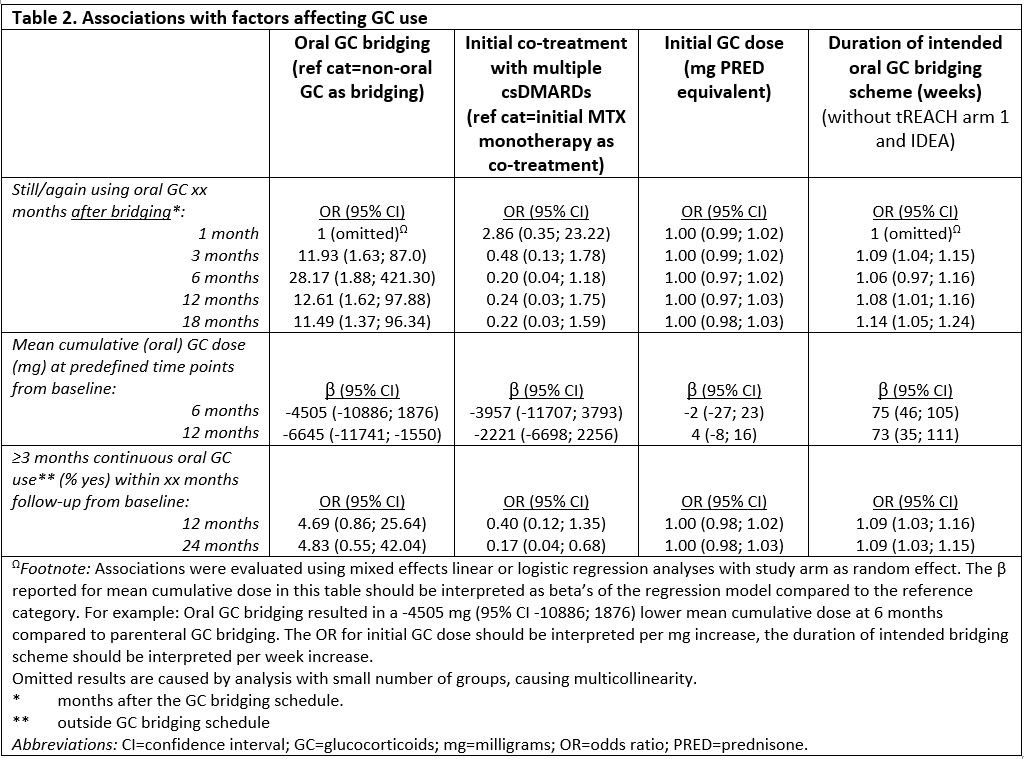
Results: Individual-level data were obtained from 1653 patients starting GC bridging therapy (orally, with a fixed multi-week dosing scheme tapering to nil, or single dosed intramuscularly or intravenously). Baseline characteristics were mostly comparable. The probability of using GC 1 month following bridging therapy was 0.18, which decreased further to 0.07 at 18 months after the end of bridging (table 1). The probability of ≥3 months continuous GC use after initial GC bridging was 0.21 within 1 year and 0.32 within 2 years of follow-up. When excluding study arms with parenteral GC bridging (IDEA intravenous arm and tREACH intramuscular arm), the probabilities on GC use and continuous GC use for ≥3 months were higher, but the cumulative dose over time was lower (table 1). Having had oral GC bridging as compared to IV or IM bridging and a longer GC bridging scheme as compared to shorter bridging schemes, were both associated with more GC use and more continuous GC use for ≥3 months. No associations were found for initial DMARD co-treatment nor for GC dose with each outcome.
Conclusion: In clinical trials with protocolized GC tapering schemes the probability of using GC after GC bridging ended is low and decreases over time. Patients who continued or restarted GC had relatively high cumulative doses (initial bridging included). A shorter bridging scheme was associated with lower cumulative doses. A non-oral route of administration and a shorter bridging scheme were associated with less frequent (continuous) GC use after bridging.
To cite this abstract in AMA style:
van Ouwerkerk L, Verschueren P, de Jong P, Emery P, Smolen J, Landewé R, Lems W, Boers M, Huizinga T, Allaart C, Bergstra S. Individual Patient Data Meta-analysis on Continued Use of Glucocorticoids After Their Use as Bridging Therapy in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/individual-patient-data-meta-analysis-on-continued-use-of-glucocorticoids-after-their-use-as-bridging-therapy-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/individual-patient-data-meta-analysis-on-continued-use-of-glucocorticoids-after-their-use-as-bridging-therapy-in-patients-with-rheumatoid-arthritis/