Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The relation between etanercept dose and clinical outcomes of juvenile idiopathic arthritis (JIA) is unclear. Most studies only evaluated doses up to 0.8 mg/kg/week (max 50mg/week),[1] but higher doses are often used off-label in clinical practice. An in-depth description of patients receiving such treatment is lacking. Here, we describe the clinical course of JIA-patients that received high-dose etanercept (1.6 mg/kg/week; max 50mg/week) as part of the Best4Kids trial.
Methods: In a single-blinded treatment-strategy trial, patients with oligoarticular JIA, RF-negative polyarticular JIA or juvenile psoriatic arthritis were randomised across three arms: (1) sequential DMARD-monotherapy (sulfasalazine or methotrexate (MTX)), (2) combination-therapy MTX+6 weeks prednisolone and (3) combination therapy MTX+etanercept.[2] A protocolised treat-to-target approach aiming for inactive disease was used during 24 months follow-up. Treatment was escalated in case of persistent disease-activity or tapered in case of inactive disease. In any treatment-arm patients could eventually escalate from regular to high-dose etanercept alongside MTX 10mg/m2/week. For comparison we studied patients who did not receive high-dose etanercept due to decisions overriding the trial-protocol by the treating pediatrician and/or the patient/parents.
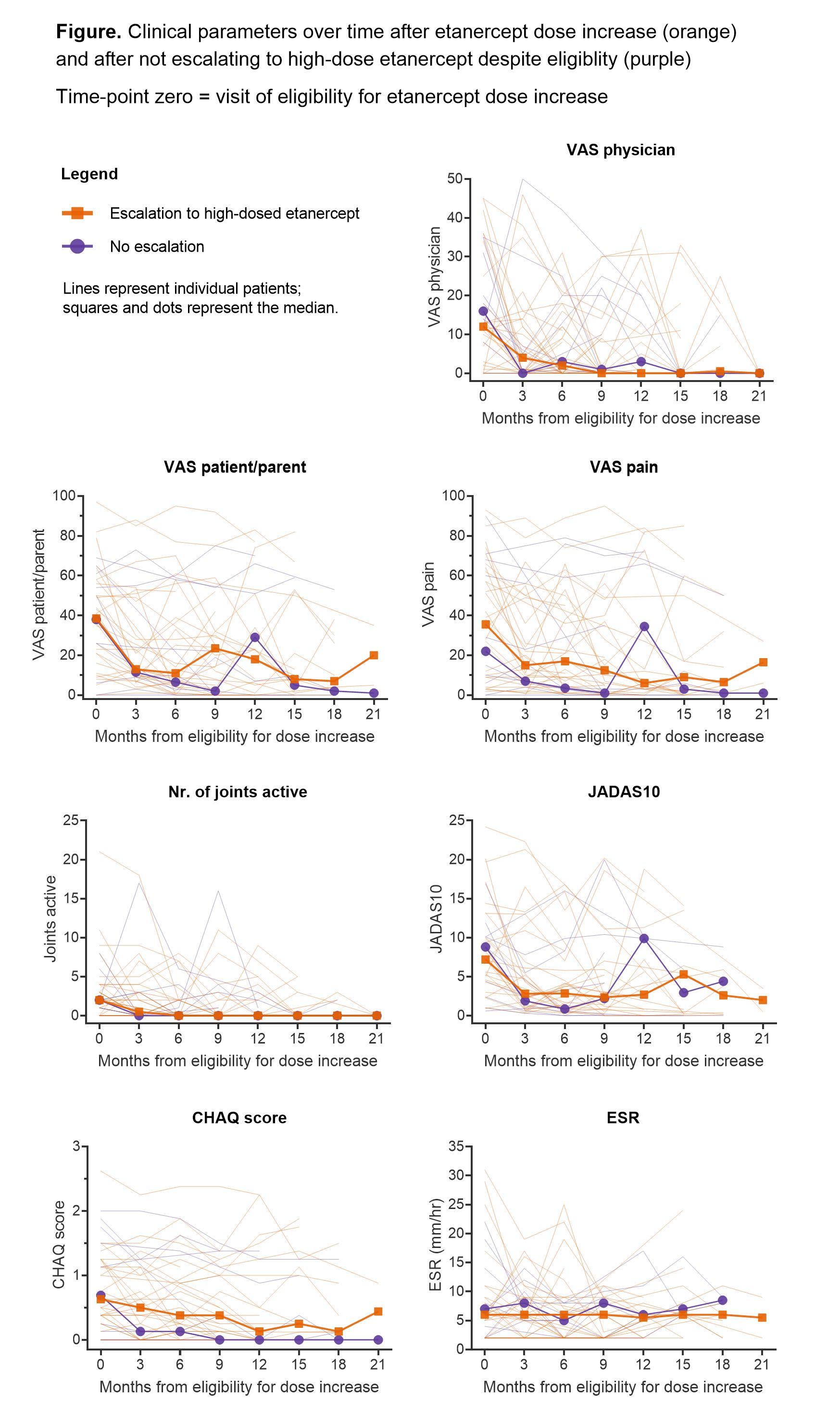
Results: Of the 94 randomised patients, 32 received high-dose etanercept (69% female, median age 6 years (IQR 4-10), median time from baseline 10 months (7-16), median dose 1.3 mg/kg/week (1.1-1.5)). Follow-up was up to 2 years from baseline (median 24.6 months). Clinical measures of disease-activity decreased largely within 3 months (Figure): median VAS-physician from 12 to 4 (p=0.022), VAS-patient/parent from 38.5 to 13 (p=0.003), VAS pain from 35.5 to 15 (p=0.030), number of active joints from 2 to 0.5 (p=0.12) and JADAS10 from 7.2 to 2.8 (p=0.008). Functional status (CHAQ-score) improved more gradually and ESR remained stable. A comparable pattern of clinical parameters over time was observed in 11 patients (73% girls, median age 8 (IQR 6-9)) who did not escalate to high-dose etanercept despite eligibility according to trial-protocol. In both the high-dose and the comparison group the percentage of patients with inactive disease 6 months after eligibility for dose-increase was 56%. In the high-dose group, 18 out of 32 patients (56%) experienced 26 infectious adverse events (AEs, on average 0.20 events per visit following dose-increase). No serious AEs (SAEs) were recorded after dose-increase. Among the 11 patients who did not receive high-dose etanercept, 4 patients (36%) subsequently experienced 5 infectious AEs (on average 0.11 events per visit); this included one SAE requiring hospitalisation.
Conclusion: Escalation to high-dose etanercept was generally followed by meaningful clinical improvement within 3 months. However, non-escalators experienced comparable improvement. These data do not suggest superior clinical outcomes after etanercept dose increase, while there was a potential trend for more (non-severe) infectious AEs. Larger studies are needed to more closely examine outcomes, adverse events and cost-effectiveness of high dose etanercept.
To cite this abstract in AMA style:
van Dijk B, Bergstra S, van den Berg M, Schonenberg-Meinema D, van Suijlekom-Smit L, van Rossum M, Koopman Y, Ten Cate R, Allaart C, Brinkman D, Hissink Muller P. Increasing the Etanercept Dose in Juvenile Idiopathic Arthritis Patients: Does It Help Reaching the Treatment Target? A Post-hoc Analysis of the Best4Kids Randomised Clinical Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/increasing-the-etanercept-dose-in-juvenile-idiopathic-arthritis-patients-does-it-help-reaching-the-treatment-target-a-post-hoc-analysis-of-the-best4kids-randomised-clinical-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-the-etanercept-dose-in-juvenile-idiopathic-arthritis-patients-does-it-help-reaching-the-treatment-target-a-post-hoc-analysis-of-the-best4kids-randomised-clinical-trial/