Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoantibodies including rheumatoid factor (RF) and antibodies to citrullinated protein antigens (ACPA) may be elevated during a period that can be termed ‘Pre-RA’. In addition, there are findings supporting that there is an expansion of autoimmunity in Pre-RA; however, this has typically been demonstrated using research grade assays and therefore the increasing positivity of commercially available ACPA and RF is less well known. As such, the purpose of this study was to evaluate how an increasing number of commercially-available autoantibodies testing during Pre-RA relates to the development of imminent RA.
Methods: Two-hundred and fifteen RA cases, each with approximately 3 pre- and 1 post-RA serum samples, were identified from the Department of Defense Serum Repository. All case samples were tested by ELISA for anti-CCP2 (IgG, Axis-Shield), CCP3 (IgG, Inova), RF-IgM, A and G (Inova). Manufacturer suggested cut-offs were used for the CCP assays, and a level present in < 2% of 156 controls was used for the RF’s. Comparison of autoantibody prevalence within a time point was performed using chi-squared and Fisher’s exact testing. Analyses of autoantibody positivity over time within cases as individual biomarkers or as an overall count were performed using analyses that account for repeated measures within subjects (e.g. ANOVA).
Results: Characteristics of the 215 cases are reported in Table 1, and the positive rates of all autoantibodies as individual tests and counts are presented in Table 2. The prevalence of all antibodies increased from the earlier pre-RA time point (a mean of 11.6 years prior to RA diagnosis) to immediately pre-RA diagnosis (a mean of 0.9 years prior to RA diagnosis) although there was no significant increase in positive rates of any of these autoantibodies when comparing the immediate pre- and post-RA diagnosis samples. There were no significant differences in rates of positivity at any time period between CCP2 and CCP3 although CCP3 had a slightly higher prevalence of positivity at earlier time points. At all times points, both CCP2 and CCP3 were positive in a greater number of subjects than any of the RF isotypes. The number of positive autoantibodies also increased over time, with the highest mean count of autoantibodies of 2.57 at the immediate pre-RA diagnosis period.
Conclusion: In these subjects CCP2 and CCP3 perform in a similar manner, although both are present in a higher number of subjects in the earlier time periods than RF. In addition, higher mean counts of antibodies are present in the immediate pre-RA period. These findings suggest that there is an expansion of autoantibody positivity across ACPA and RF assays. Furthermore, testing for these widely-available assays and identifying a high number of positives may be useful to identify subjects in Pre-RA who may imminently transition to clinically-apparent and classifiable RA
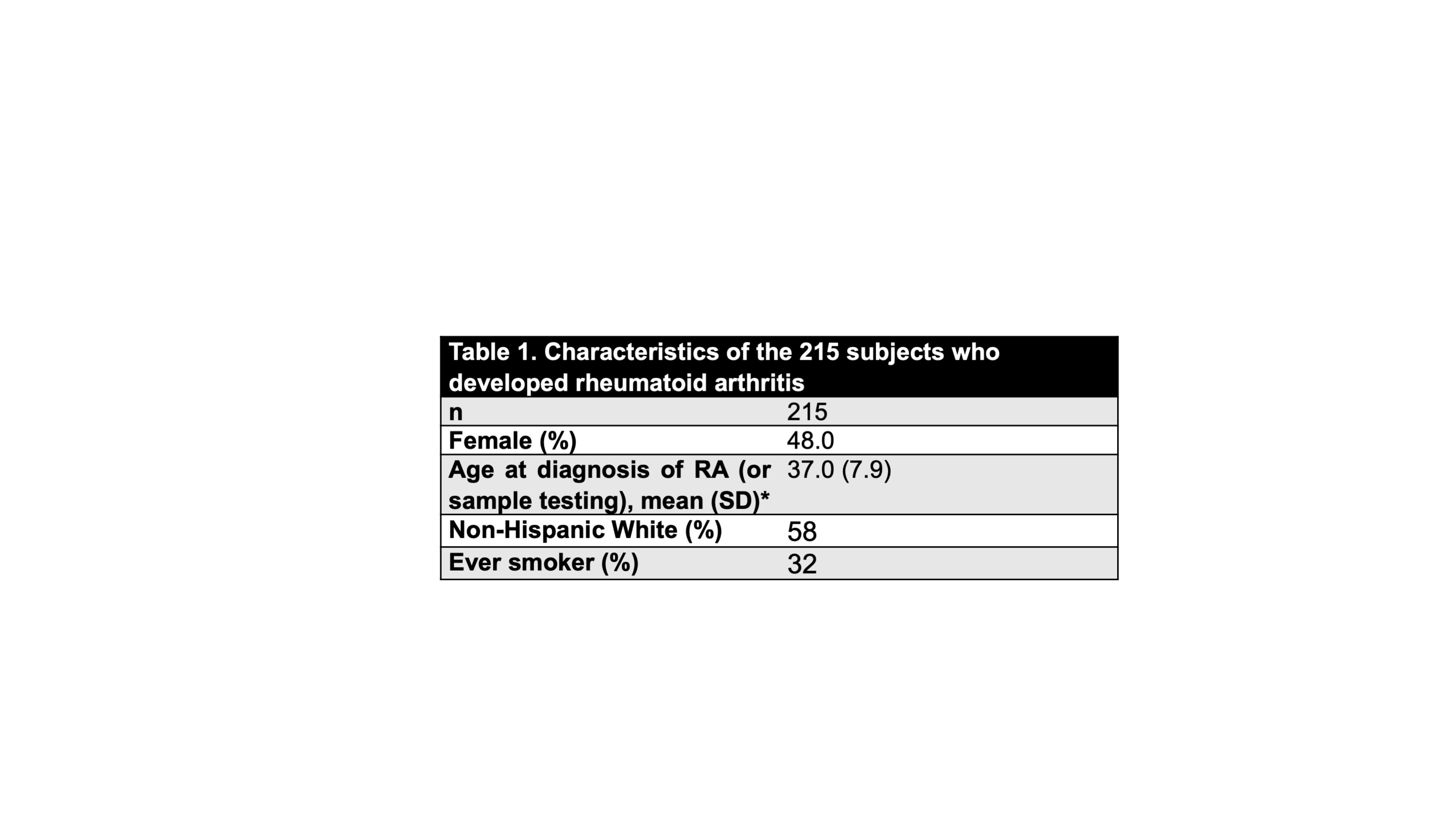 Table 1. Characteristics of the 215 subjects who developed rheumatoid arthritis
Table 1. Characteristics of the 215 subjects who developed rheumatoid arthritis
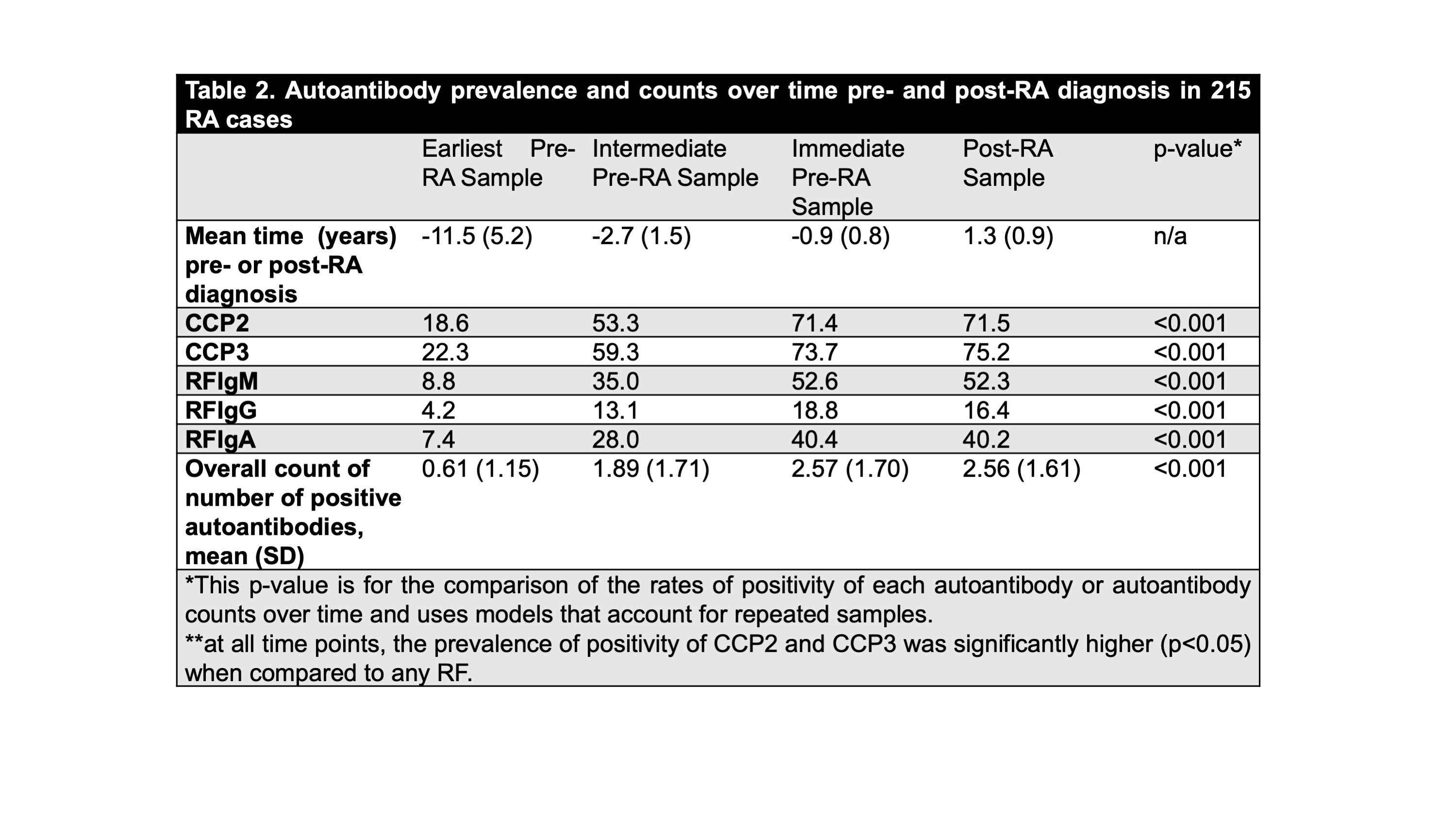 Table 2. Autoantibody prevalence and counts over time pre- and post-RA diagnosis in 215 RA cases
Table 2. Autoantibody prevalence and counts over time pre- and post-RA diagnosis in 215 RA cases
To cite this abstract in AMA style:
Greenblatt H, Mikuls T, Edison J, Feser M, Parish M, Moss L, Mewshaw E, D. Deane K. Increasing Autoantibody Positivity During Pre-RA Is Associated with the Imminent Development of Classifiable RA [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/increasing-autoantibody-positivity-during-pre-ra-is-associated-with-the-imminent-development-of-classifiable-ra/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-autoantibody-positivity-during-pre-ra-is-associated-with-the-imminent-development-of-classifiable-ra/
