Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: A growing number of older adults are living with autoimmune rheumatic diseases (AIRDs) as the global population is aging. And yet, they remain underrepresented in pharmacologic randomized clinical trials (RCTs). This has led to a gap in the evidence-base for the care of older adults with AIRDs. Understanding existing age-related inclusion practices in AIRD RCTs is essential to address this gap. This systematic review evaluates the inclusion of older adults in pharmacologic RCTs of AIRDs.
Methods: We conducted a systematic review (PROSPERO CRD42023457996) using PubMed, EMBASE, CINAHL Complete, Europe PMC, ClinicalTrials.gov, and the International Clinical Trials Registry Platform. We included completed phase II–IV pharmacologic RCTs with published results from 2010–2023. For rheumatoid arthritis (RA), the search began in 2017, as a prior review covered earlier studies.¹We included the following AIRDs: ANCA-associated vasculitis (AAV), axial spondyloarthritis (ax-SpA), giant cell arteritis (GCA), idiopathic inflammatory myopathies (IIM), polymyalgia rheumatica (PMR), psoriatic arthritis (PsA), reactive arthritis (ReA), RA, systemic lupus erythematosus (SLE), and systemic sclerosis (SSc). Ax-SpA, PsA, and ReA were grouped as spondyloarthritis (SpA), and GCA and PMR were analyzed together (GCA/PMR).We included RCTs enrolling adults ≥18 years of age, except for AAV and SLE, in which a lower age threshold (≥15 years) was selected. Title/abstract screening, full-text review, and data extraction were performed independently by two reviewers. Conflicts were resolved a by a third reviewer. Mean age and standard deviation (SD) of participants by AIRD were calculated using methods developed by Palmowski et al¹ and compared to ages reported in historical observational cohorts for each AIRD.
Results: We identified 515 RCTs including 152,185 participants (Table 1). RA, SpA, and SLE accounted for 80% of RCTs and 94% of participants. Higher female representation was noted in RCTs for GCA/PMR, SLE, SSc, and IIM. Most trials were conducted in North America, Europe, and Australasia.Age-related exclusion was common, with 56.4% of RCTs applying upper age limits, most frequently a cut-off at 70 years (Table 2). Only 33.3% of RCTs explicitly reported data on older adults. Across multiple AIRDs—including SLE, SSc, SpA, GCA/PMR, and RA—the mean participant age was lower than that observed in representative cohort studies.
Conclusion: Older adults are underrepresented in RCTs across all AIRDs. Upper age limit exclusion criteria are commonly used, restricting the inclusion of the older adult population. These findings highlight the limitations in the generalizability and applicability of RCT results to inform/guide the care of older adults. There is an urgent need to promote the inclusion of older adults with AIRD in RCTs and observational studies, in line with National Institute of Health’s Inclusion Across the Lifespan Policy, to improve delivery of care and outcomes in this growing population.ReferencesPalmoswki, et al. Semin Arthritis Rheum. 2019.
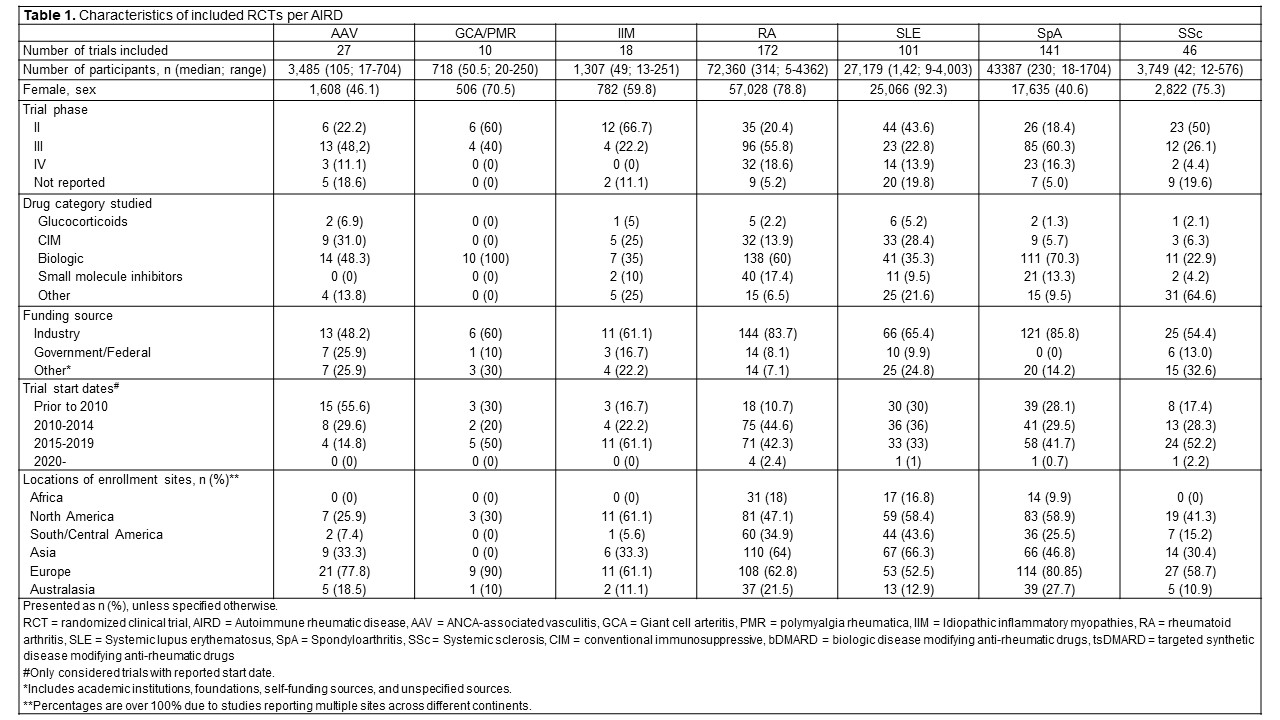 Table 1. Characteristics of included RCTs per AIRD
Table 1. Characteristics of included RCTs per AIRD
.jpg) Table 2. Mean ages and inclusion of older adults in RCTs per AIRDs
Table 2. Mean ages and inclusion of older adults in RCTs per AIRDs
.jpg) Figure 1. PRISMA flowchart for included randomized clinical trials
Figure 1. PRISMA flowchart for included randomized clinical trials
To cite this abstract in AMA style:
Carpio Tumba M, Lomanto Silva R, Sung L, Pedraza-Arévalo L, Gupta S, Gwak J, Mohamadi A, Louden D, Stovall R, Singh N, Saygin D, Lieber S, Lee J, Sattui S. Inclusion of Older Adults in Pharmacologic Randomized Controlled Clinical Trials of Autoimmune Rheumatic Diseases: A Systematic Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/inclusion-of-older-adults-in-pharmacologic-randomized-controlled-clinical-trials-of-autoimmune-rheumatic-diseases-a-systematic-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/inclusion-of-older-adults-in-pharmacologic-randomized-controlled-clinical-trials-of-autoimmune-rheumatic-diseases-a-systematic-review/
