Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In pre‑licensure clinical trials, numerical differences in gout cases between the recombinant zoster vaccine (RZV) and placebo groups have been observed. However, real-world evidence is limited. Thus, we aimed to estimate the risk of incident gout following RZV exposure using real-world data from a large population of older US adults.
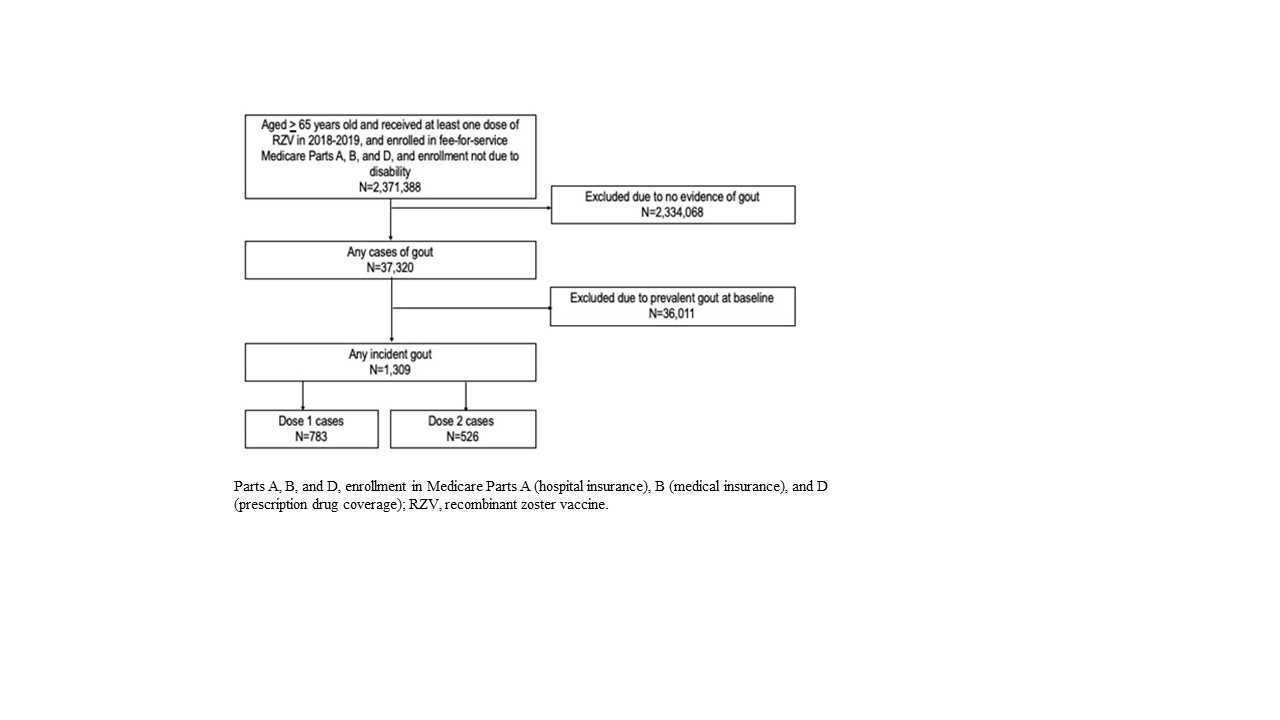
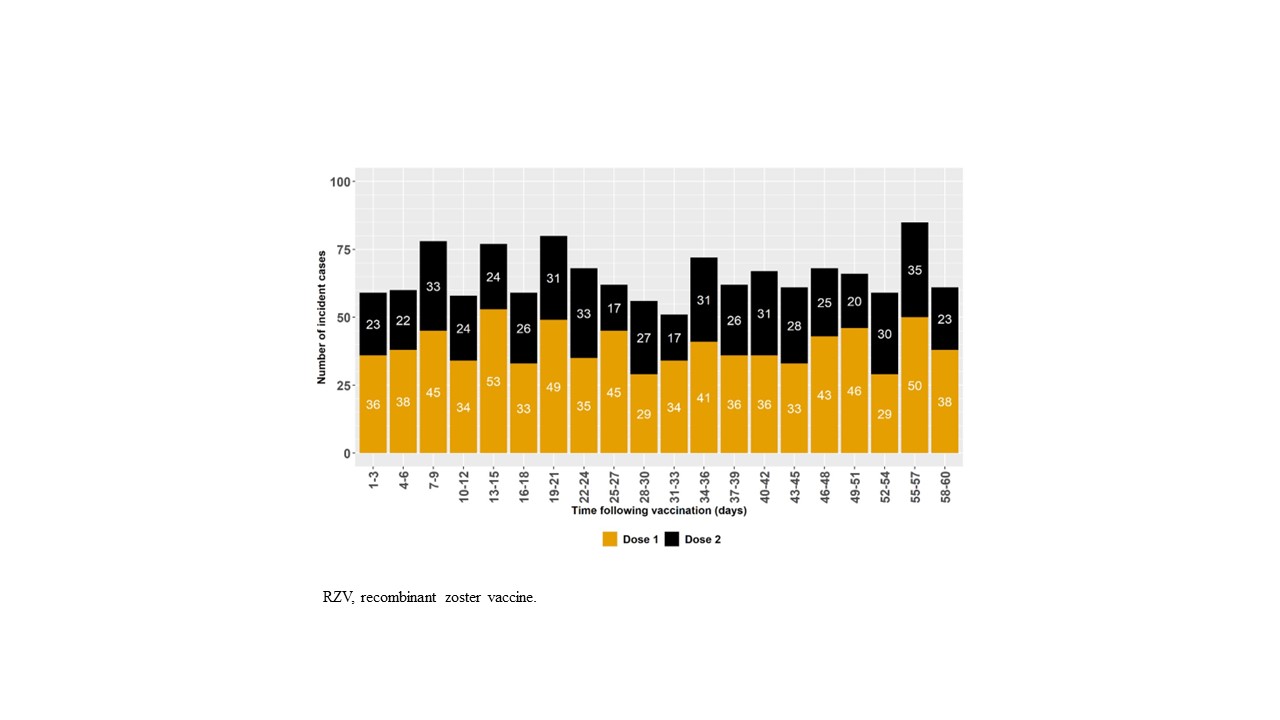
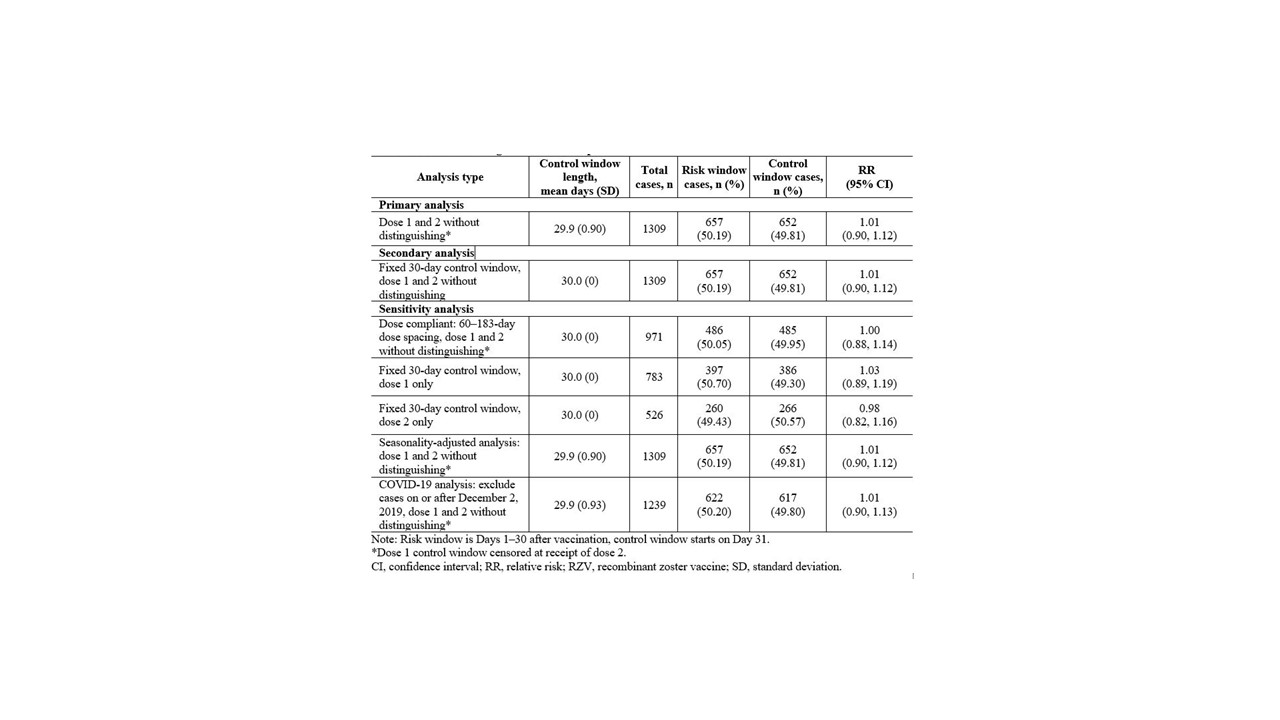
Methods: Using the Centers for Medicare and Medicaid Services Chronic Conditions Warehouse database, a case-only, self-controlled risk interval design was implemented to assess the risk of incident gout in US adults aged ≥65 years. We included adults who were enrolled in fee-for-service Medicare Parts A, B, and D and received at least one dose of the two-dose RZV regimen in 2018–2019 (Figure 1). Incident gout cases in the risk (Days 1–30 following vaccination) or control (Days 31–60 following vaccination) window (Figure 2) were defined by a gout diagnosis in an inpatient or outpatient setting followed by receipt of allopurinol, colchicine, probenecid, or febuxostat within 3 months with no prior evidence of gout diagnosis or medication in the 365 days preceding the incident gout case. Baseline descriptive characteristics in the 12 months up to RZV exposure included age, sex, calendar year–month of RZV vaccination, concomitant preventive immunizations, comorbidities, and health care service utilization. Conditional Poisson regression models estimated the relative risk (RR) of incident gout following any RZV dose in a primary (with censoring at receipt of dose 2) and secondary (30-day fixed control window without censoring) analysis. Sensitivity analyses included 1) a two-dose recipient subgroup with 60–183-day dose spacing; 2) dose 1- and dose 2-specific analysis; 3) a seasonality-adjusted analysis; and 4) a COVID-19 sensitivity analysis excluding incident gout cases on or after December 2, 2019. A temporal scan tested for significant clustering of incident gout cases after vaccination.
Results: We identified 1,309 incident gout cases (Figure 1) who were predominantly White (87%; n=1137) and male (61%; n=801), and aged 70–79 years (55%; n=723). Chronic kidney disease (41%; n=531), diabetes mellitus (36%; n=473), and ischemic heart disease (33%; n=433) were common comorbidities. The majority had no prior hospitalizations (87%; n=1,135) or Emergency Department visits (77%; n=1,013), and 9% (n=121) received concomitant influenza vaccine. Of the 1,309 incident gout cases, 1,074 (82%) received two RZV doses. The 783 incident gout cases after dose 1 and the 526 incident gout cases after dose 2 occurred equally in the risk and control windows (Figure 2). In the primary analysis (Table 1), the RR of incident gout was 1.01 (95% confidence interval: 0.90, 1.12). The secondary analysis and all sensitivity analyses produced similar results. The temporal scan did not detect significant clustering of incident gout cases over the 60-day follow-up.
Conclusion: The findings show no statistically significant increased risk of incident gout following receipt of RZV in US adults aged ≥65 years.
To cite this abstract in AMA style:
dosReis S, Zhang C, Amill-Rosario A, Johnson A, Lee H, Spence O, Oraichi D, Seifert H, Franck V, Gamble S, Yun H. Incident Gout After Recombinant Zoster Vaccination in Adults Aged ≥65 Years in the USA [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/incident-gout-after-recombinant-zoster-vaccination-in-adults-aged-%e2%89%a565-years-in-the-usa/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incident-gout-after-recombinant-zoster-vaccination-in-adults-aged-%e2%89%a565-years-in-the-usa/