Session Information
Date: Monday, November 11, 2019
Title: Pediatric Rheumatology – ePoster II: SLE, Juvenile Dermatomyositis, & Scleroderma
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ), a medication used to treat Systemic Lupus Erythematosus (SLE), Discoid Lupus Erythematosus (DLE) and Sjogren’s Syndrome (SS), reduces disease flare-ups, organ damage, and increases rates of remission1,2. While HCQ is relatively safe, recent research among adults has shown its long-term use to be associated with an increased risk of developing retinopathy2. The link between HCQ exposure during childhood and the incidence of retinopathy has not been established. This study describes a contemporary cohort of pediatric patients who initiated treatment with HCQ for SLE, DLE or SS prior to their 18th birthday and assesses the incidence of retinopathy.
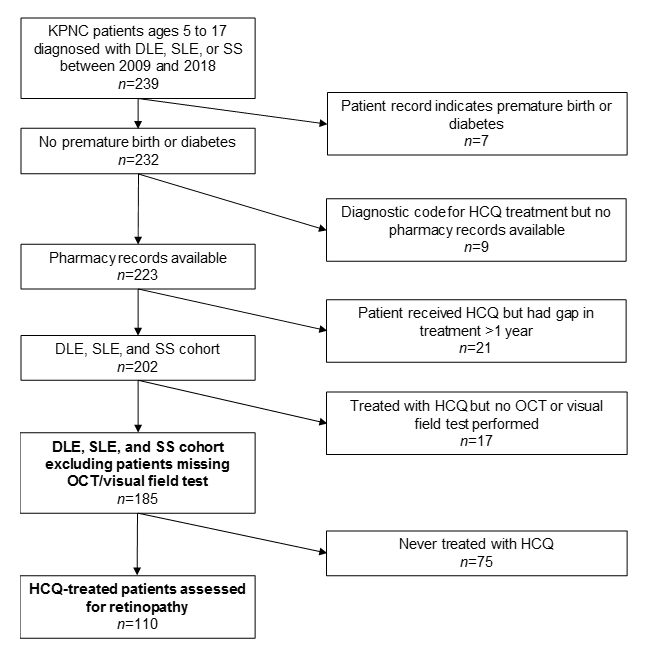
Methods: This is a retrospective descriptive analysis of patients receiving treatment within Kaiser Permanente Northern California (KPNC), who were diagnosed with SLE, DLE or SS between the ages of 5-17 years, and did not have evidence of premature birth or diabetes. Demographic information, as well as the age at diagnosis, were obtained from the electronic health record. The pharmacy database was queried for HCQ use. Chart review was performed to ensure patients met the American College of Rheumatology criteria for SLE and DLE, and the proposed criteria for juvenile SS. Further chart review was performed to determine evidence of retinopathy, defined by vision loss on the Humphrey Visual Field testing (HVF) and/or retinal thinning on the Spectral-domain optical coherence test (SD-OCT). Of the 232 patients who met the initial criteria, we further excluded those with no pharmacy record (n=9), those with treatment gaps greater than one year (n=21) and those with no evidence of a completed HVF or SD-OCT (n=17), resulting in a final study cohort of 185 patients and 110 patients with continuous HCQ exposure (Figure 1).
Results: The 185 individuals who met the initial study criteria had a mean age at diagnosis of 13.8 years (standard deviation: 3.01) and 80.0% were female. Of this cohort, 14.1% identified as non-Hispanic white, 18.9% black, 34.6% Hispanic non-black, 22.7% Asian/Pacific Islander and for 9.7%, the race/ethnicity was unknown. There were 75 patients who were never treated with HCQ, 63 who received less than three years of treatment, and 47 who were treated for three or more years (Table 1). HCQ treatment duration ranged from 0.1 to 9.6 years, with a median of 2.5 years and an interquartile range between 1.5 and 5.5 years. There were no demographic variations according to HCQ treatment duration. No patients developed retinopathy.
Conclusion: To the best of our knowledge, this is the first study to evaluate the incidence of retinopathy in a pediatric population exposed to HCQ for the treatment of SLE, DLE and SS. None of the patients developed retinopathy. Establishing a low risk of retinopathy for children taking HCQ is clinically relevant for treatment discussions in pediatric rheumatology, between providers, patients, and families. Further studies are needed to investigate risk in longer-term use.
References:
- Groot, N, de Graeff N, Avcin T, et al. Ann Rheum Dis 2017.; 76: 1788-1796.
- Melles, R.B.,Marmor, M.F. The Risk of Toxic Retinopathy in Patients on Long-term Hydroxychloroquine Therapy. JAMA Ophthalmology 2014; 132 (12): 1453-1460.
To cite this abstract in AMA style:
Patrizi S, Stram D, Weintraub M, Aminoff A. Incidence of Retinopathy in Individuals Who Initiated Hydroxychloroquine Therapy During Childhood [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/incidence-of-retinopathy-in-individuals-who-initiated-hydroxychloroquine-therapy-during-childhood/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-retinopathy-in-individuals-who-initiated-hydroxychloroquine-therapy-during-childhood/