Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Systemic Sclerosis & Related Disorders II: Clinical Research
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Although prevalence of interstitial lung disease (ILD) in systemic sclerosis (SSc) and associated risk factors are established, less is known about its incidence and the risk factors associated with new onset of ILD following a negative baseline. We aimed to (1) estimate the annual incidence of ILD and (2) identify risk factors for new onset of ILD in baseline negative patients.
Methods: SSc patients classified according to the 2013 ACR/EULAR criteria from the EUSTAR database, with absence of ILD on high resolution computed tomography (HRCT) at baseline and having at least 1 follow-up visit were included. Patients with pulmonary arterial hypertension diagnosed by right heart catheterization were excluded. Based on follow-up HRCT, our population was divided into patients with new onset of ILD (incident group) and patients who remained ILD negative (negative group). Incidence of SSc-ILD was calculated as rate per 1000 person-years and presented starting from the first visit at the center. Prediction models were built to identify risk factors for ILD onset, with logistic regression for the 1-year and Cox regression for the long-term observation. Known predictors of new onset of ILD were chosen as covariates from previous literature and based on expert opinion.
Results: We identified 5336 ILD negative patients at baseline. New onset of ILD occurred in 1080 (20.2%) cases with median of 3.8 (IQR 1.6-7.3) years follow-up.
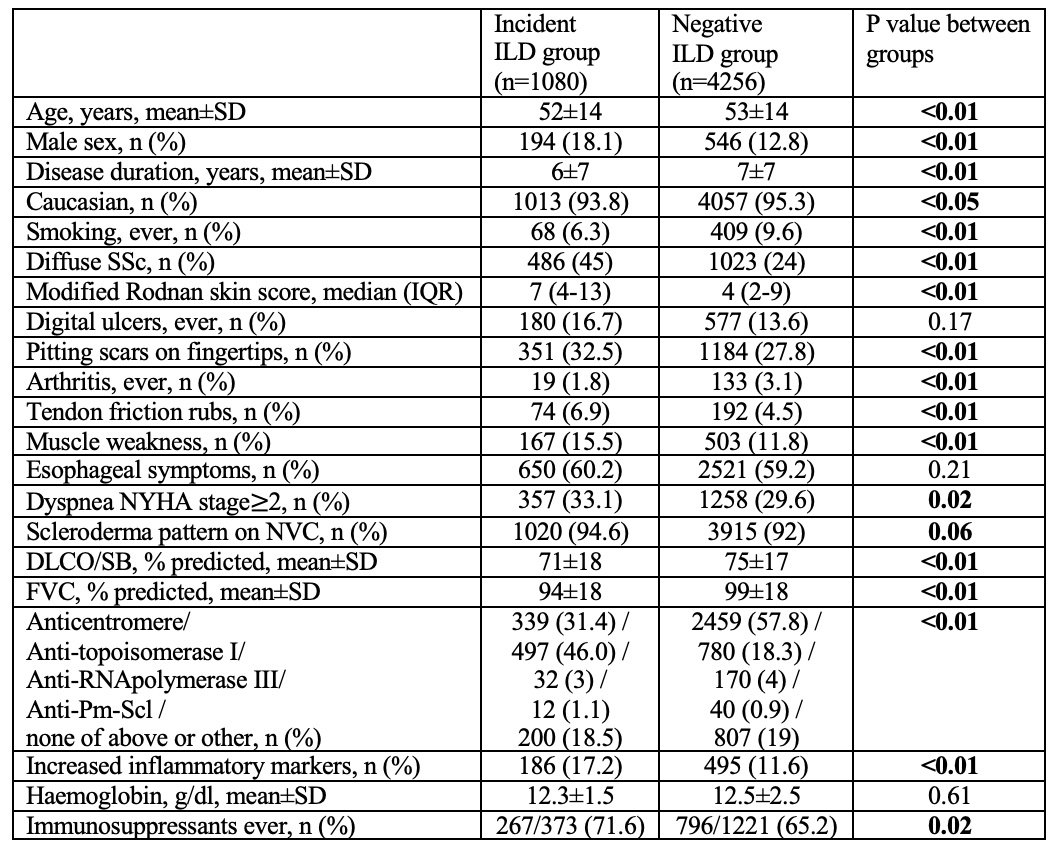
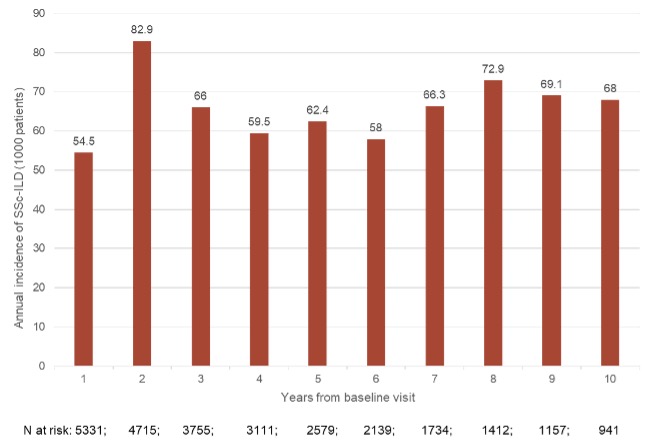
The overall ILD incidence rate was 74.0 (95% CI: 68.5-79.5) per 1000 person-years. There was a continuous detection of new onset of ILD during the longitudinal observation, up to 10 years from baseline (incidence rate 54.5-82.9 per 1000 person-years; Figure 1). The baseline characteristics of incident and negative groups are shown in Table 1.
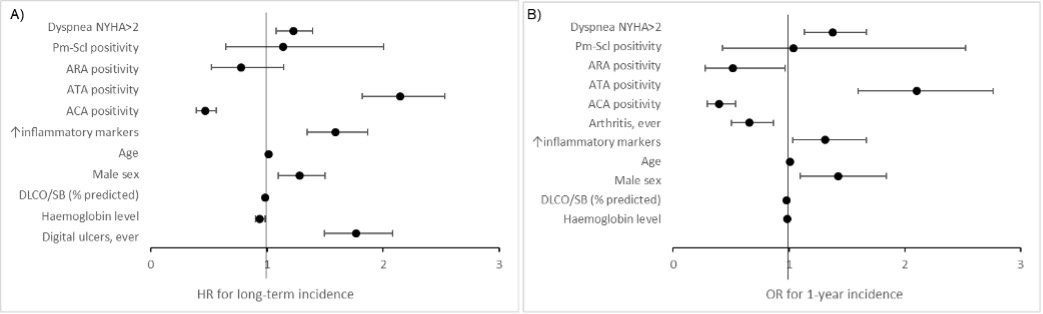
In the analysis of long-term incidence, new onset of ILD was independently predicted by dyspnea NYHA stage≥2 (HR 1.23, 95% CI1.08-1.40), male sex (HR 1.28, 95% CI1.10-1.50), age (HR 1.02, 95% CI 1.01-1.02), DLCO/SB% (HR 0.99, 95% CI 0.98-0.99), elevated inflammatory markers (HR 1.59, 95% CI 1.35-1.87), haemoglobin level (HR 0.94, 95% CI: 0.90-0.98), anti-topoisomerase I antibody (HR 2.15, 95% CI 1.82-2.53), anti-centromere antibody (HR 0.47, 95% CI0.39-0.56,) and digital ulcers ever (HR1.77, 95% CI: 1.49-2.08) (Figure 2 A). Surprisingly, the incidence of new onset of ILD was independent from disease duration.
The analysis for 1-year incidence included 13339 yearly follow-ups from 4067 baseline negative patients. All risk factors of new onset of ILD identified in the analysis of long-term incidence analysis, except for digital ulcers ever, were confirmed (Figure 2B).
When exposure to immunosuppressants was forced into the two models, this was not retained as a protective factor.
Conclusion: ILD can appear at any time after SSc diagnosis, with similar incidence during the disease course. We identified risk factors for new onset of ILD both at 1-year and long-term follow-up. Therefore, SSc patients should be screened following a negative baseline HRCT.
To cite this abstract in AMA style:
Petelytska l, Velauthapillai A, Tofani L, Hachulla E, Müller-Ladner U, Siegert E, ALLANORE Y, Riemekasten G, Bergmann C, Becvar R, Solanki K, Anic B, Rednic S, Stamenkovic B, Stamp L, Distler J, Vonk M, de Vries-Bouwstra J, Hoffmann-Vold A, Matucci Cerinic m, Distler O, Bruni C. Incidence and Risk Factors for New Onset of Interstitial Lung Disease in Systemic Sclerosis: A EUSTAR Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/incidence-and-risk-factors-for-new-onset-of-interstitial-lung-disease-in-systemic-sclerosis-a-eustar-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-and-risk-factors-for-new-onset-of-interstitial-lung-disease-in-systemic-sclerosis-a-eustar-analysis/