Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: During the height of the COVID-19 pandemic in the United States, tixagevimab/cilgavimab (Evusheld), a combination of monoclonal antibodies directed against the SARS-CoV2 spike protein, received emergency use authorization (EUA) for use as pre-exposure prophylaxis (PrEP) against COVID-19. However, little is known regarding real-world experience with PrEP in patients with systemic autoimmune rheumatic diseases (SARDs). We aimed to determine the incidence of and risk factors for breakthrough COVID-19 after tixagevimab/cilgavimab among patients with SARDs.
Methods: We conducted a retrospective cohort study of patients with SARDs at a large U.S. healthcare system. We identified all patients who received tixagevimab/cilgavimab as PrEP between 1/2/2022 and 11/16/2022. The index date was the date of the first dose of tixagevimab/cilgavimab. We collected demographics, medications, prior COVID-19, and SARS-CoV2 spike antibody levels prior to index date from electronic query. SARD diagnoses, the presence of additional PrEP indications (e.g., prior organ transplant, active cancer treatment), and COVID-19 course were confirmed by medical record review. The primary outcome was breakthrough COVID-19 confirmed by polymerase chain reaction or an antigen test reported to a physician. Censoring occurred at earliest of breakthrough COVID-19, death, or end of study (November 16, 2022). We used multivariable Cox regression models adjusting for baseline factors to identify risk factors for breakthrough COVID-19.
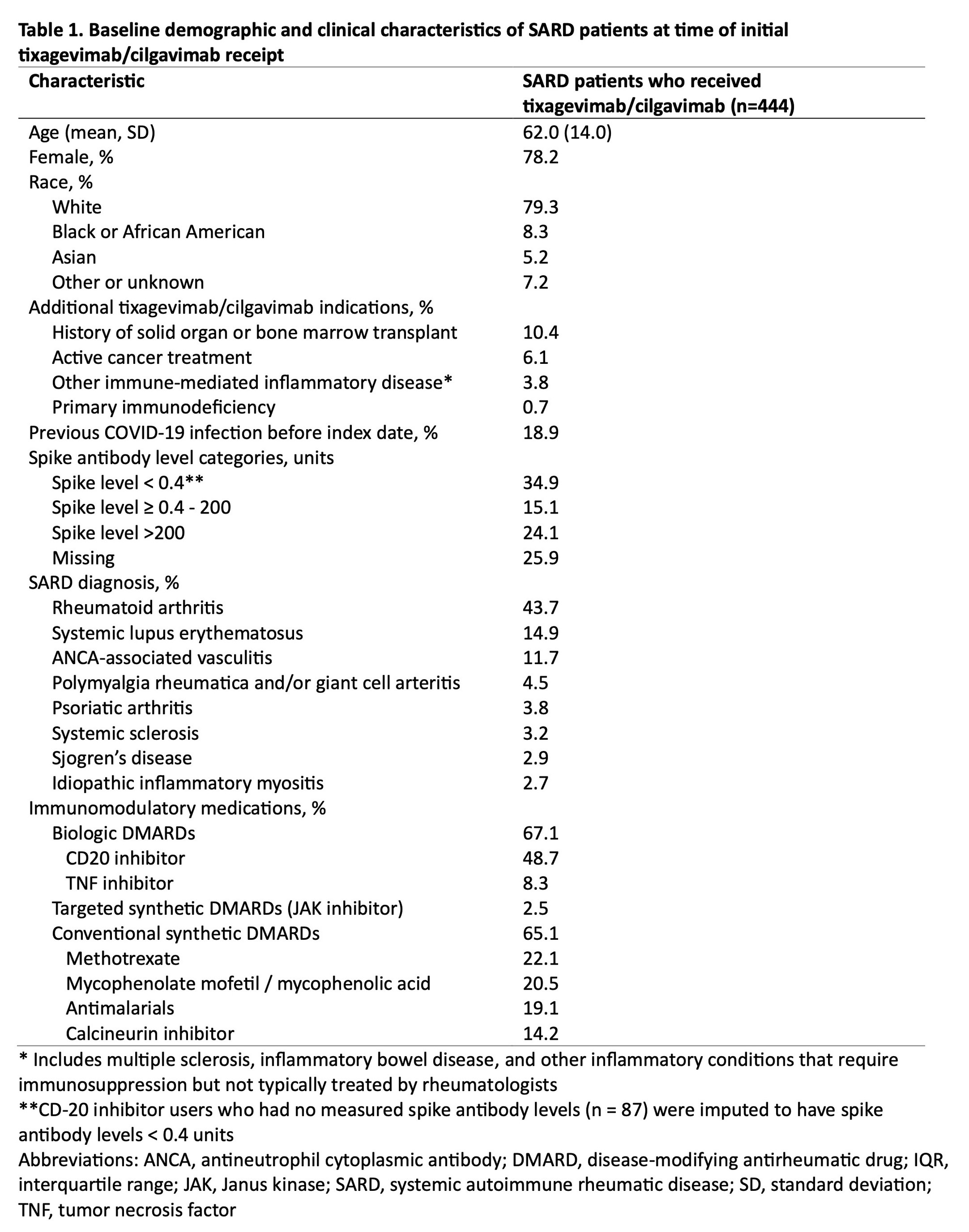
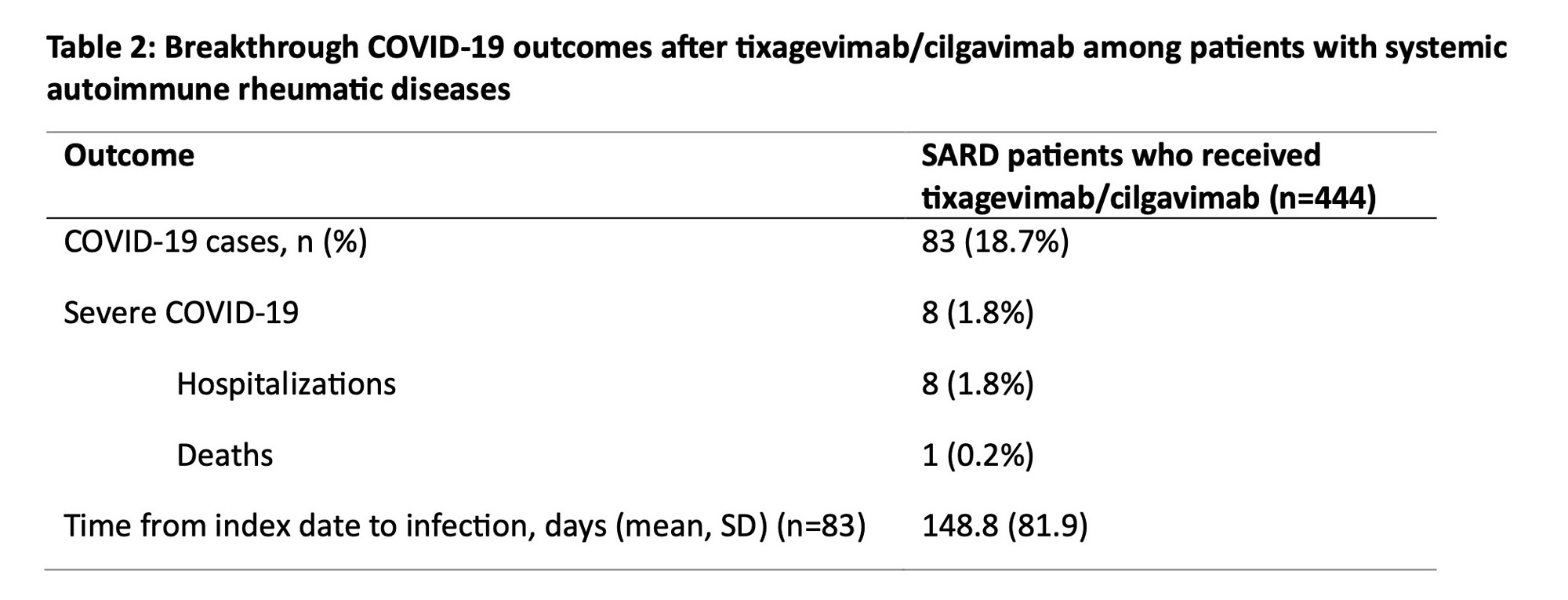
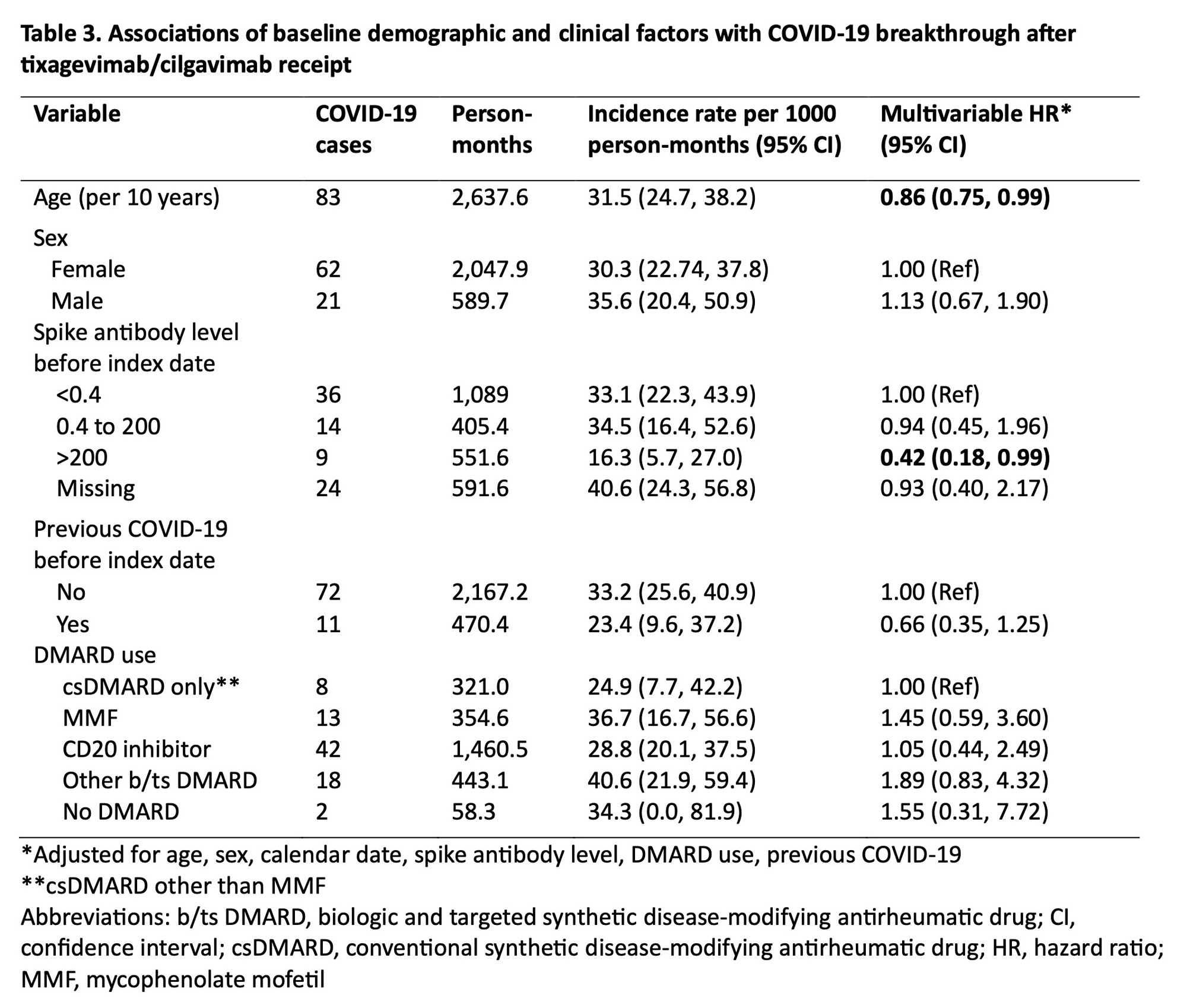
Results: We identified 444 patients with SARDs who received tixagevimab/cilgavimab (mean age 62.0 years, 78.2% female, 79.3% white) (Table 1). The most common SARD diagnoses were rheumatoid arthritis (43.7%), systemic lupus erythematosus (14.9%), and ANCA-associated vasculitis (11.7%). There were 83 (18.7%) breakthrough COVID-19 cases, of which 8 (1.8%) required hospitalization and 1 (0.2%) died (Table 2). The overall incidence rate was 31.5 per 1000 person-months (95% CI 24.70, 38.24). Older age was inversely associated with breakthrough COVID-19 (adjusted hazard ratio [aHR] 0.86 per 10 years, 95% CI 0.75, 0.99). Higher baseline spike antibody levels were associated with lower risk of breakthrough COVID-19 (aHR 0.42, 95% CI 0.18, 0.99 for spike antibody levels >200 units vs. < 0.4 units). CD20 inhibitor users had a similar risk of breakthrough COVID-19 (aHR 1.05, 95% CI 0.44, 2.49) compared to patients on conventional synthetic DMARDs (Table 3).
Conclusion: In this study spanning the period of viral neutralizing abilityfor tixagevimab/cilgavimab, we found that patients with SARDs had frequent breakthrough COVID-19 after PrEP, but the proportion of severe COVID-19 was low. Further, we found no associations between DMARD type, including anti-CD20 inhibitors, with the risk of breakthrough COVID-19. Older age and higher baseline spike antibody level were protective against breakthrough infection, highlighting the continued need for a multimodal approach (e.g. shielding behaviors, vaccinations) to prevent COVID-19 in this vulnerable population as newer generation PrEP monoclonal antibodies against SARS-CoV2 are being developed.
To cite this abstract in AMA style:
Kawano Y, Wang X, Patel N, Qian G, Kowalski E, Bade K, Vanni K, Medicines Partnership (AMP): RA/SLE A, Williams Z, Cook C, Srivatsan S, Wallace Z, Sparks J. Incidence and Risk Factors for Breakthrough COVID-19 After Tixagevimab/Cilgavimab Among Patients with Systemic Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/incidence-and-risk-factors-for-breakthrough-covid-19-after-tixagevimab-cilgavimab-among-patients-with-systemic-autoimmune-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-and-risk-factors-for-breakthrough-covid-19-after-tixagevimab-cilgavimab-among-patients-with-systemic-autoimmune-rheumatic-diseases/