Session Information
Date: Monday, November 14, 2022
Title: T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Immune-mediated inflammatory arthritis (IMIA) is a group of diseases characterized by chronic synovitis including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and spondyloarthritis (SpA). Some disease modifying antirheumatic drugs (DMARDs) have demonstrated efficacy in several of these diseases (e.g. TNFα inhibitors) while others have not (e.g. IL-17A inhibitors in RA). Furthermore, within each disease there are patient subgroups experiencing different responses to the same drug, which underlines the need for further elucidation of pathogenic processes to guide personalized in IMIAs. Here we investigated if clinical features and synovial transcriptional signature could predict in vitro efficacy of specific DMARDs across different IMIAs.
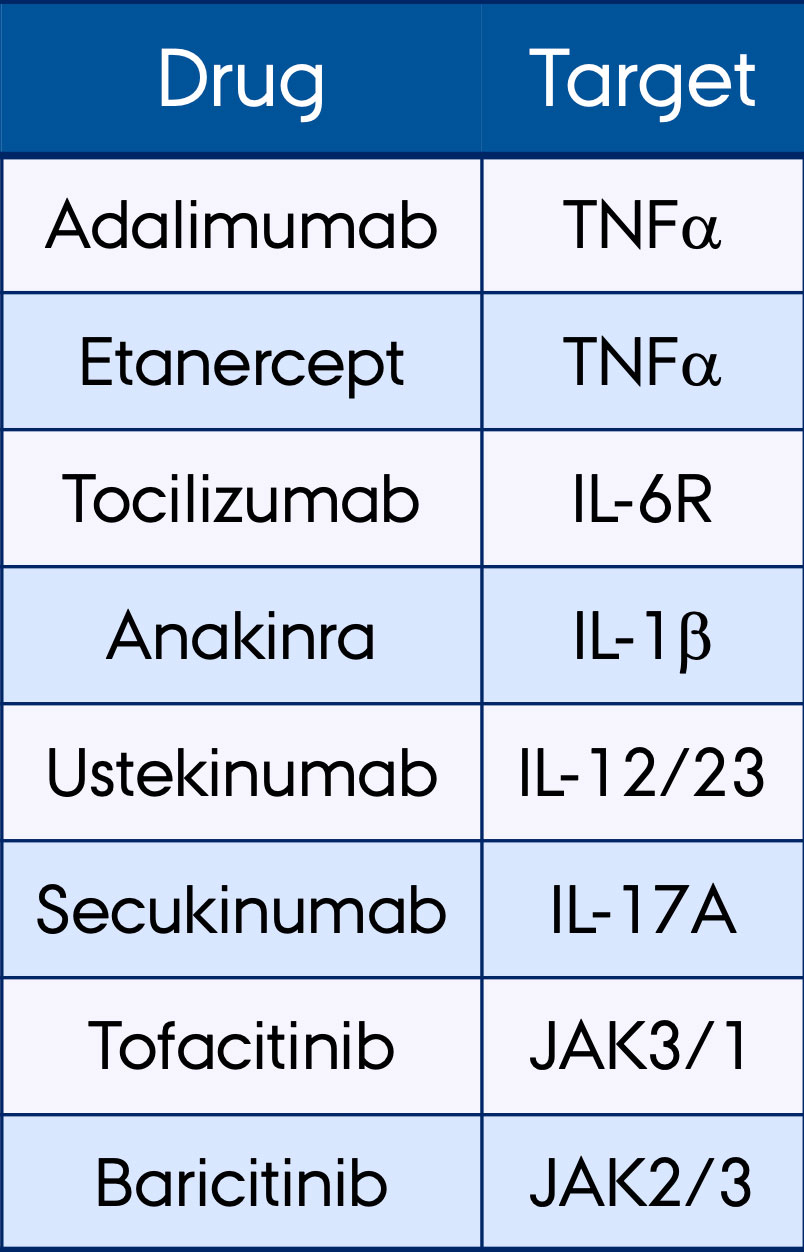
Methods: Synovial fluid mononuclear cells (SFMCs) from patients with SpA (n=13), RA (n=16), PsA (n=13), juvenile idiopathic arthritis (JIA) (n=7) or undifferentiated arthritis (n=5) were included. SFMCs from each patient were cultured for 48 hours (drugs listed in Table 1). Culture medium and DMSO were used as negative controls. In vitro drug response was assessed by measuring monocyte chemoattractant protein 1 (MCP-1) by ELISA. SFMC baseline gene expression of 270 genes related to inflammation was measured using the NanoString nCounter Human Inflammation V2 Panel. Drug responses (ΔMCP-1) were compared with clinical diagnosis, serostatus in RA patients, HLA-B27 status in SpA patients, DAS28-CRP score, years since diagnosis, failed modes of action and transcriptional signatures.
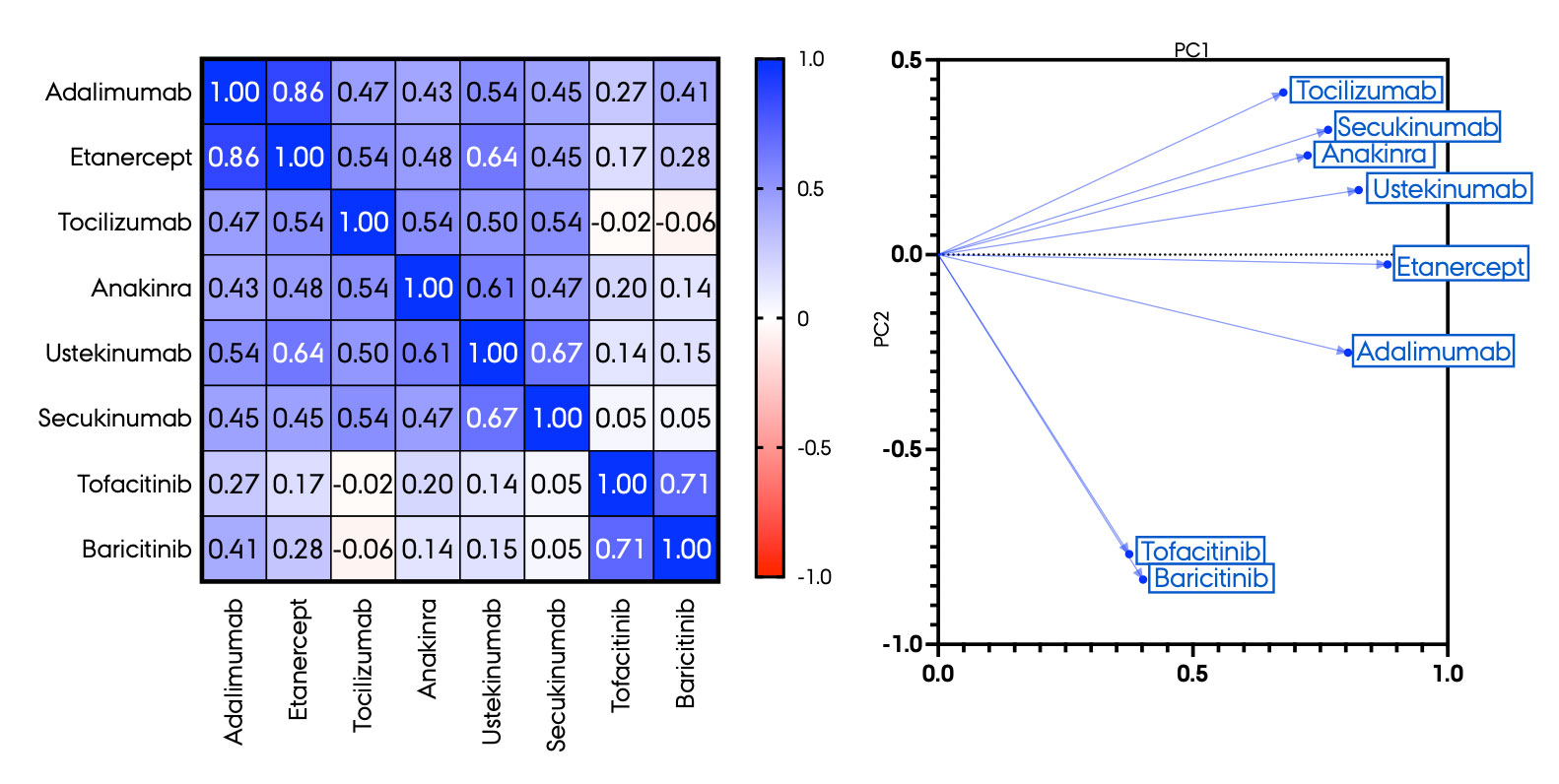
Results: DMARDs with a similar molecular target had comparable effects on ΔMCP-1. The TNFα inhibitors adalimumab and etanercept (r=0.86) had similar drug responses, and so had the JAK inhibitors tofacitinib and baricitinib (r=0,71). In contrast, drugs with different modes of action had diverse responses (Figure 1). High HLA-DRA gene expression associated with high responses to TNFα inhibitors (both p< 0.05). JAK inhibitors reduced MCP-1 secretion significantly more in SFMCs from seropositive than seronegative RA patients (p< 0.05). Drug response was greater in SFMCs from patients with low DAS28-CRP scores (DAS28-CRP < 2.5) compared with patients with a high DAS28-CRP score (DAS28-CRP >5.2) for TNFα inhibitors, tocilizumab and JAK inhibitors (all p< 0.05). Patients clustered with either an overall high or an overall low response to almost all DMARDs, or with a significant response to only one or a few of the DMARDs. Transcriptional signature of patient SFMCs primarily responding to TNFα inhibitors was different compared with the signature of patient SFMCs primarily responding to JAK inhibitors. Diagnosis, HLA-B27 status, failed modes of action and years since diagnosis did not affect ΔMCP-1 in this in vitro setup.
Conclusion: Similarities and differences in mode of action for 8 different DMARDs were captured in drug response as measured by MCP-1 change in SFMC cultures from 54 patients with inflammatory arthritis. We were able to identify interesting differences in gene expression when comparing cells responding to TNF inhibitors to cells responding to JAK inhibitors. Drug responses were only to a minor extent associated with clinical features.
To cite this abstract in AMA style:
Brüner M, Ahmadov U, Nielsen M, Kristensen L, Kragstrup T. In Vitro Response to Disease Modifying Antirheumatic Drugs Link Molecular Drug Target with Synovial Transcriptional Signature [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/in-vitro-response-to-disease-modifying-antirheumatic-drugs-link-molecular-drug-target-with-synovial-transcriptional-signature/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-vitro-response-to-disease-modifying-antirheumatic-drugs-link-molecular-drug-target-with-synovial-transcriptional-signature/