Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Pediatric Rheumatology – Basic Science (1927–1930)
Session Type: Abstract Session
Session Time: 3:45PM-4:00PM
Background/Purpose: Cytokine storm syndromes (CSS) are frequently fatal complications of a variety of oncologic, rheumatic, and infectious diseases. Many patients with CSS possess heterozygous missense mutations in genes related to the familial CSS, hemophagocytic lymphohistiocytosis (HLH). We recently identified missense mutations in a potentially novel CSS gene, DOCK8, among CSS patients. During the pandemic, we screened SARS-CoV-2 CSS patients (COVID-19 & MIS-C) for DOCK8 mutations, and studied the role of DOCK8 as a CSS gene using a murine model of HLH.
Methods: To date, 20 adult patients enrolled in a COVID-19 CSS clinical trial at UAB had whole genome sequencing. Four (20%) had rare heterozygous DOCK8 mutations (3 missense, 1 intronic) (Table). DOCK8 missense mutations were also identified in 6 children (UAB & Northwell) hospitalized with MIS-C (Table). DOCK8 mutations, or wild-type (WT) sequence controls, were introduced into human NK-92 cells by FOAMY virus transduction. WT and mutant DOCK8-expressing NK-92 cells were incubated with K562 target cells and compared for cytolysis and degranulation (CD107a) by flow cytometry. DOCK8 knock-out (KO) mice were compared to WT mice using an LCMV viral model of familial HLH (Jordan MB et al. Blood 2004;104:735). Following infection, mice were tracked for weight and changes in peripheral blood cell counts and cytokines as previously described (Rood JE et al. Blood 2016;127:426). After 8 days of infection, mice were euthanized and quantified for splenomegaly (weight) and liver pathology (scored by microscopy). Appropriate statistical approaches were employed for comparing nominal and ordinal data sets, respectively.
Results: Introduction of all DOCK8 missense mutations (from COVID-19 adults and children with MIS-C) into NK-92 cells resulted in diminished NK cell cytolysis and degranulation (Figure 1). In comparison to WT mice, DOCK8 KO mice displayed increased splenomegaly and prolonged weight loss (Figure 2). In addition, thrombocytopenia was statistically significantly pronounced in the DOCK8 KO mice, as was increased serum interferon-gamma (Figure 2). Histopathology revealed statistically significant liver endothelial cell activation in the DOCK8 KO mice (Figure 2).
Conclusion: DOCK8 partial dominant-negative missense mutations are present in CSS patients with COVID-19 and MIS-C and likely are risk factors for CSS development. A murine familial HLH model demonstrates a requirement for the presence of DOCK8 to prevent a hyper-inflammatory CSS-like disease state.
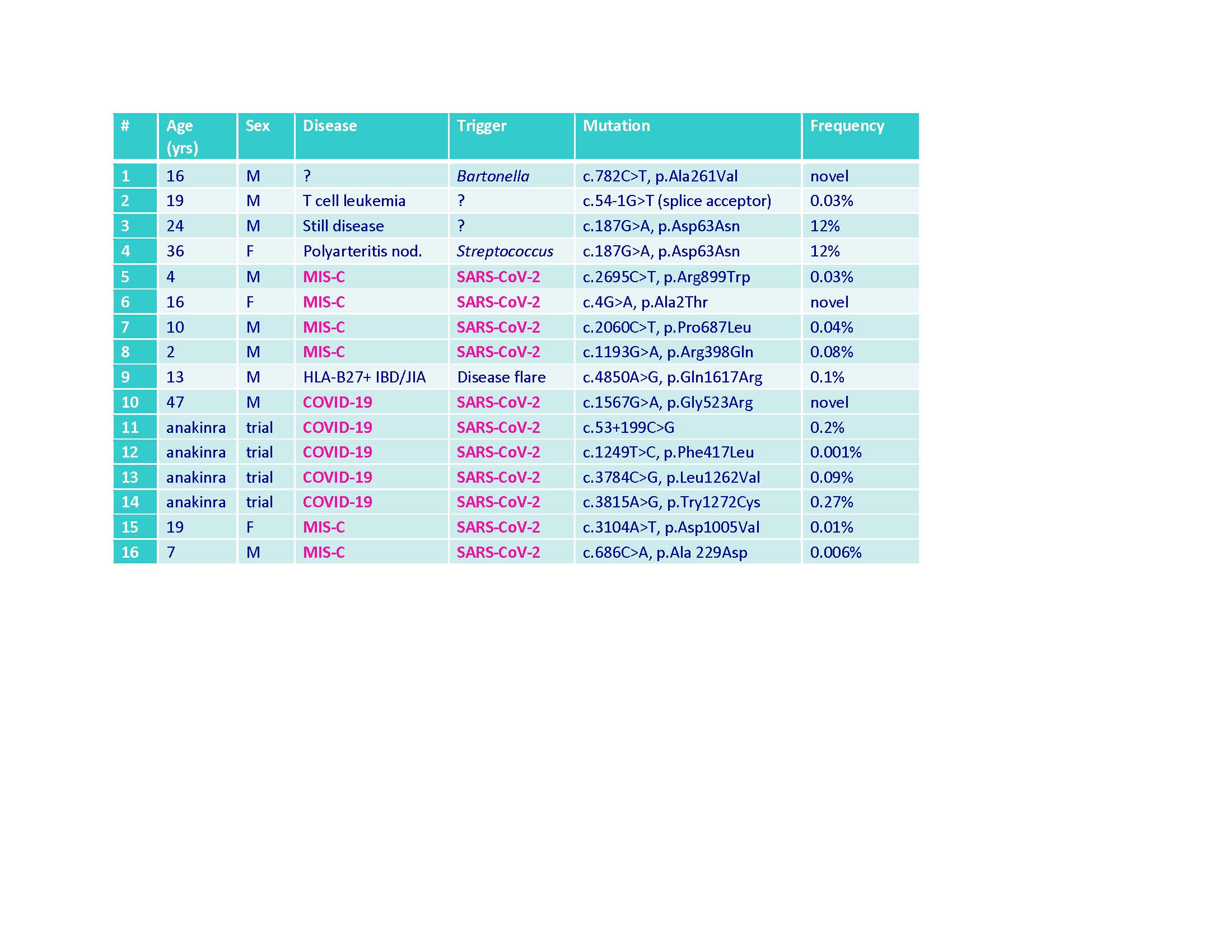 Table. DOCK8 mutations identified in children and adults with various cytokine storm syndromes, including COVID_19 and MIS-C.
Table. DOCK8 mutations identified in children and adults with various cytokine storm syndromes, including COVID_19 and MIS-C.
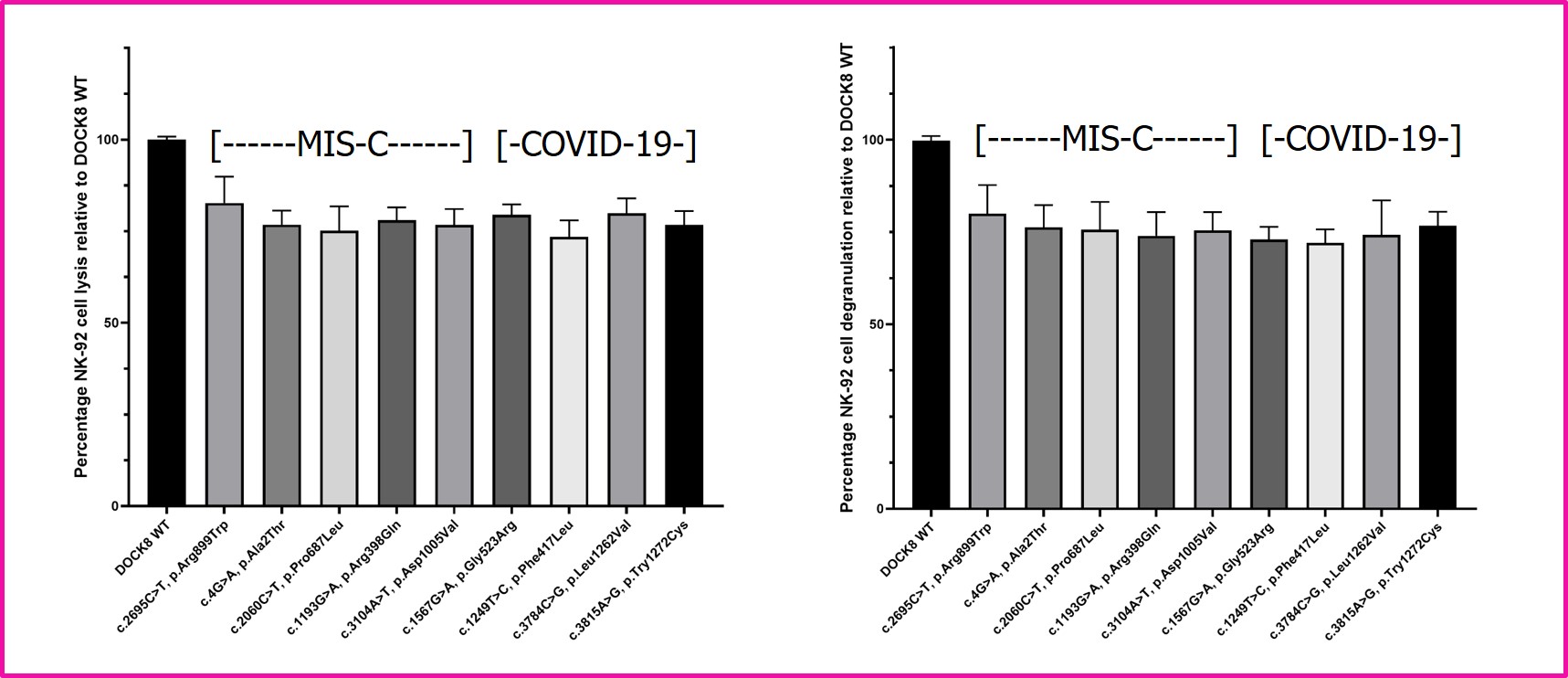 Figure 1. Functional analyses of DOCK8 patient mutations. Human NK-92 natural killer cells were infected with FOAMY viruses expressing DOCK8 wild-type (WT) or patient missense mutations. NK-92 cells were sorted by flow cytometry based on co-expression of green fluorescent protein. LEFT: NK-92 cell lytic activity versus K562 target cells was assessed for each patient DOCK8 missense mutation and compared to control (WT). Mean percentages +/- SEM are presented relative to WT (n=3). RIGHT: Degranulation of NK-92 cells stimulated by incubation with K562 target cells was assessed for each patient DOCK8 missense mutation by detection of cell surface CD107a by flow cytometry in comparison to WT control. Mean percentages +/- SEM are presented relative to WT (n=3). Studies were conducted as previously detailed (Zhang M et al. J. Immunol. 2016;196:2492).
Figure 1. Functional analyses of DOCK8 patient mutations. Human NK-92 natural killer cells were infected with FOAMY viruses expressing DOCK8 wild-type (WT) or patient missense mutations. NK-92 cells were sorted by flow cytometry based on co-expression of green fluorescent protein. LEFT: NK-92 cell lytic activity versus K562 target cells was assessed for each patient DOCK8 missense mutation and compared to control (WT). Mean percentages +/- SEM are presented relative to WT (n=3). RIGHT: Degranulation of NK-92 cells stimulated by incubation with K562 target cells was assessed for each patient DOCK8 missense mutation by detection of cell surface CD107a by flow cytometry in comparison to WT control. Mean percentages +/- SEM are presented relative to WT (n=3). Studies were conducted as previously detailed (Zhang M et al. J. Immunol. 2016;196:2492).
 Figure 2. LCMV infection model of cytokine storm syndrome in DOCK8 deficient mice. Wild-type (WT) or DOCK8-/- mice were infected with LCMV as described (Rood JE et al. Blood 2016;127:426). A. Daily body weight as a percentage of initial body weight is graphed for B6 WT (closed circles) and DOCK8-/- (open circles) with means + SEM (n=9 per group). B. Splenomegaly was assessed by spleen weight after euthanization 8 days post-infection (n=9 per group). C. Platelet counts were assessed prior to (closed circles) and 8 days post-infection (open circles) for WT (left) and DOCK8-/- mice (right) (n=8 per group). D. Serum interferon-gamma levels were assessed at day 8 post-infection for WT and DOCK8-/- animals (n=10 per group). E. Endothelial cell activation was assessed by a pathology based scoring system for WT and DOCK8-/- mice (n=10 per group). F. Representative H&E stains of liver pathologic specimens for WT (left) and DOCK8-/- (right) mice 8 days after infection.
Figure 2. LCMV infection model of cytokine storm syndrome in DOCK8 deficient mice. Wild-type (WT) or DOCK8-/- mice were infected with LCMV as described (Rood JE et al. Blood 2016;127:426). A. Daily body weight as a percentage of initial body weight is graphed for B6 WT (closed circles) and DOCK8-/- (open circles) with means + SEM (n=9 per group). B. Splenomegaly was assessed by spleen weight after euthanization 8 days post-infection (n=9 per group). C. Platelet counts were assessed prior to (closed circles) and 8 days post-infection (open circles) for WT (left) and DOCK8-/- mice (right) (n=8 per group). D. Serum interferon-gamma levels were assessed at day 8 post-infection for WT and DOCK8-/- animals (n=10 per group). E. Endothelial cell activation was assessed by a pathology based scoring system for WT and DOCK8-/- mice (n=10 per group). F. Representative H&E stains of liver pathologic specimens for WT (left) and DOCK8-/- (right) mice 8 days after infection.
To cite this abstract in AMA style:
Cron R, Zhang M, Chu N, Absher D, Bridges J, Schnell A, Vagrecha A, Lozinsky S, Acharya S, Levy C, Chatham W, Behrens E. In Vitro and In Vivo Evidence for DOCK8 as a Risk Allele for Cytokine Storm Syndrome, Including COVID-19 and MIS-C[abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/in-vitro-and-in-vivo-evidence-for-dock8-as-a-risk-allele-for-cytokine-storm-syndrome-including-covid-19-and-mis-c/. Accessed .
« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/in-vitro-and-in-vivo-evidence-for-dock8-as-a-risk-allele-for-cytokine-storm-syndrome-including-covid-19-and-mis-c/
