Session Information
Date: Monday, November 13, 2023
Title: (1082–1099) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Nephritis remains one of the most devastating complications of Systemic lupus erythematosus (SLE). Lupus nephritis (LN) significantly reduces overall survival to approximately 88% at 10 years. The ACR guideline suggests that the frequency of follow-up visits is determined by the activity and severity of the disease and its complications. Current recommendations for monitoring lupus nephritis include UA (urine analysis with microscopy) and urine Protein/creatinine ratio (UPCR). This test should be obtained every 6 months for patients with SLE without active LN, every 3 months in patients with non-active LN, and every month in patients with active LN. Our overall goal was to increase the appropriate monitoring of LN in patients with SLE in our rheumatology clinic.
Methods: This quality improvement project retrospectively analyzed the medical records of 60 patients diagnosed with SLE who were seen at the rheumatology clinic at the University of Florida in Jacksonville, FL, from January 2019 to December 2019. Patients were classified as SLE without LN, non-active LN, and with active LN. The frequency of performed UA and UPCR, were recorded in each subject. After identifying baseline values, we worked with clinicians and clinic support staff on intervention. Interventions included educating fellows, faculty, and staff about current guidelines for LN screening and monitoring, creating flyers, and generating a panel order in the electronic health system (EPIC) for SLE, including UA and UPCR tests. A subsequent retrospective chart review of 60 patients was performed after 12 months to analyze the impact of interventions.
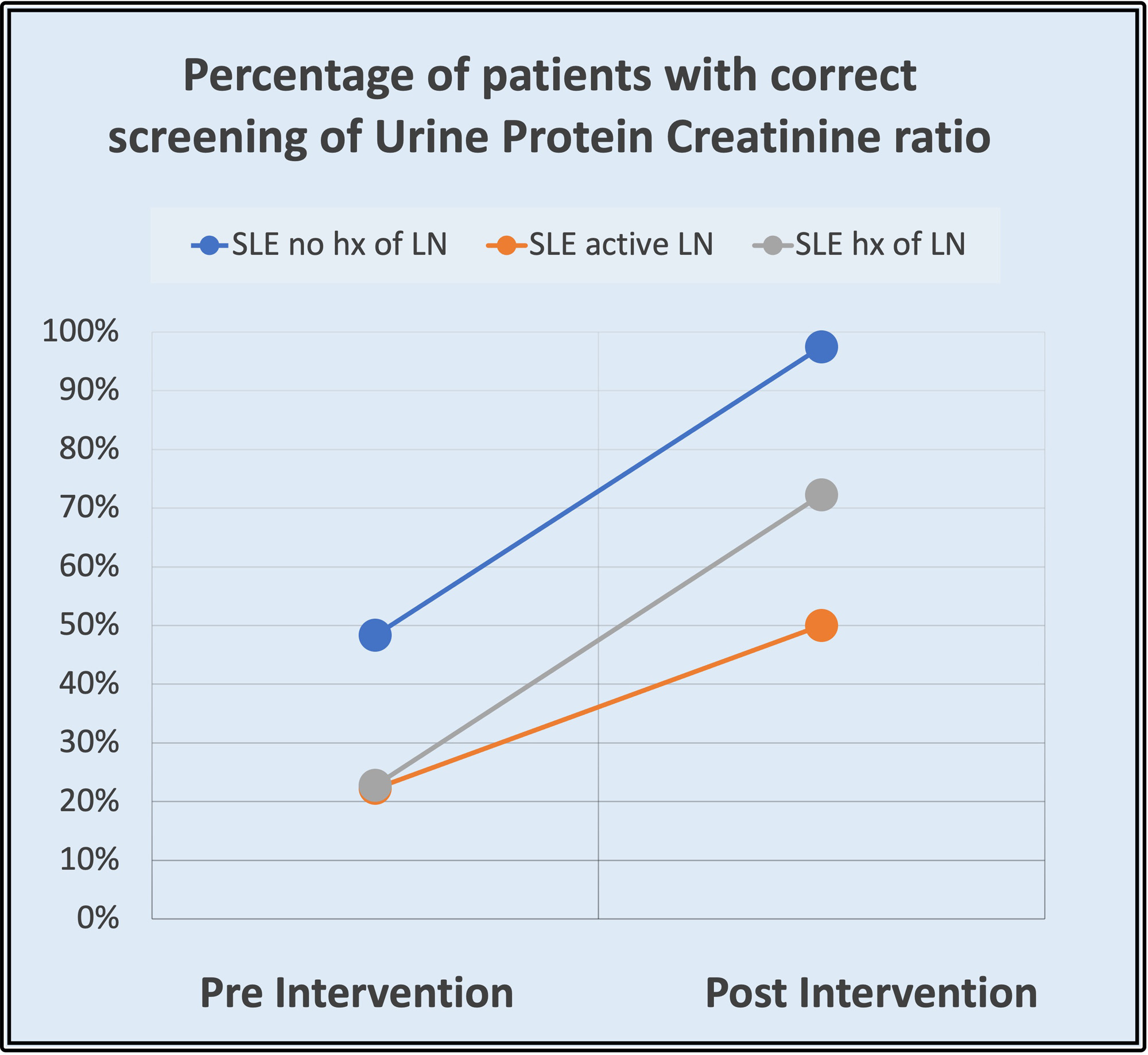
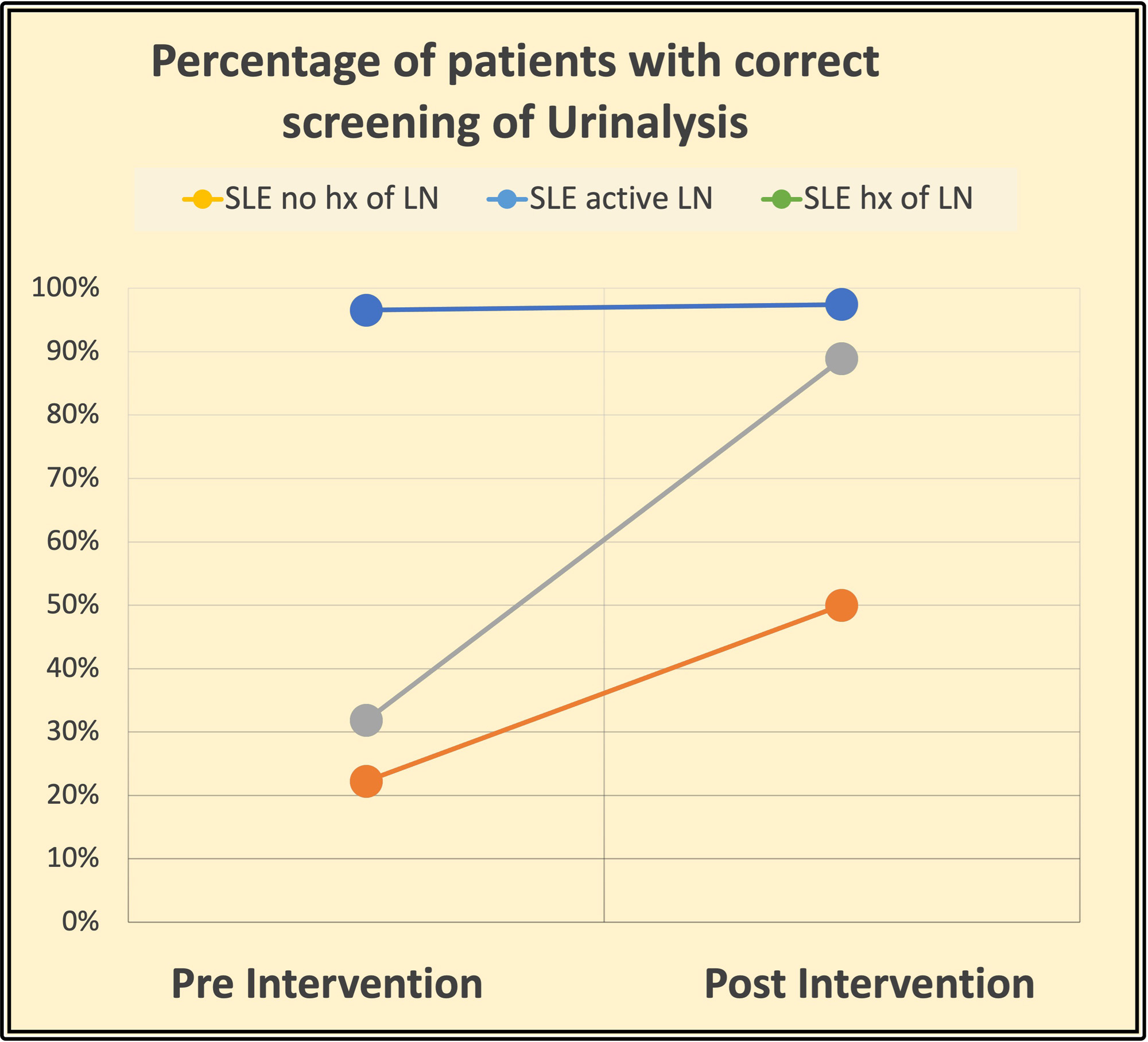
Results: Initial data showed the need for improvement in monitoring/screening laboratory parameters (UPCR and UA) for LN in our patients with SLE. Preintervention analysis showed that less than 50% of patients were correctly monitored for UPCR in the three groups, and less than 40% were monitored for UA in patients with active LN and with a history of LN as recommended per ACR guidelines. Just one group of patients: SLE patients with no LN history had a high UA monitoring rate (97%). After the intervention, monitoring/screening for LN in patients with SLE improved: Regarding UPCR, preintervention results were 48% (no history of LN), 23% (history of LN), and 22% (active LN), and it increased to 97%, 72% and 50% respectively. Adequate monitoring of UA preintervention results were 97% (no history of LN), 32% (history of LN), and 22% (active LN, and postintervention 97%, 89%, 50%. A separate chart review of those patients suggested that compliance of patients is the major issue of proper testing intervals.
Conclusion: LN is associated with increased mortality and morbidity in SLE patients. Survival of SLE has improved in the past few decades with advanced treatment, including early diagnosis of LN. ACR developed recommendations for screening LN and follow-up tests. Adherence to proper screening may help renal survival by treating exacerbations on a timely basis. This study employed various interventional methods to increase the screening of Urinalysis and proteinuria. However, patient compliance remains a challenge.
To cite this abstract in AMA style:
Otalora Rojas L, S Kaeley G, Thway M. Improving Screening of Lupus Nephritis in Patients with Systemic Lupus Erythematous [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improving-screening-of-lupus-nephritis-in-patients-with-systemic-lupus-erythematous/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-screening-of-lupus-nephritis-in-patients-with-systemic-lupus-erythematous/