Session Information
Date: Sunday, October 26, 2025
Title: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Despite guideline recommendations, recombinant zoster vaccine (RZV) uptake remains low among immunocompromised individuals younger than 50 years of age, notably those with rheumatoid arthritis (RA). Previous institutional efforts, including patient and physician education, demonstrated increased willingness to receive the vaccine but the impact was limited. Additional strategies to increase uptake were warranted. Our aim was to increase RZV uptake among RA patients ages 18-49 from 10% to 15% by January 2025 through a targeted partnership with specialty pharmacy.
Methods: We defined our baseline cohort as RA patients ages 18-49 years seen at a tertiary care clinic from July 2023 to December 2023. Prior patient and physician educational interventions increased baseline RZV uptake from 6.7% to 10.3% (January 2024 to May 2024). Root cause analysis identified vaccine access as a major barrier. Starting June 2024, we partnered with Stanford Specialty Pharmacy, that is located near the clinic, to develop a coordinated vaccine delivery pathway aligned with rheumatology visits. Our improvement team included clinic nurses, patient care coordinators (PCC), pharmacists, and rheumatologists. A comprehensive workflow was established including communication regarding eligibility, RZV prescription with obtaining prior authorization, pre-visit vaccine counseling, and RZV administration on date of clinic visit (Figure 1). In November 2024, a separate workflow was created to coordinate the second vaccine dose within 2-6 months post-first dose (Figure 2). To minimize barriers to vaccine access, validated parking was provided in collaboration with Stanford Medicine Transportation Services. The primary outcome was the proportion of eligible patients to receive at least 1 dose of RZV following intervention during June 2024 to January 2025. The secondary outcome was completion of 2 dose series following implementation (November 2024). Barriers to RZV were collected from telephone encounters between patient and pharmacist.
Results: Among the baseline cohort (n=137), 15.3% received at least one RZV dose between June 2024 and January 2025, meeting the intervention goal. Following implementation of the workflow for dose #2 of RZV, 5 of 9 eligible patients (55.5%) completed the two-dose series by May 2025. Common barriers to vaccination included reproductive considerations (n=5) including in vitro fertilization, pregnancy, or breastfeeding as well as insurance authorization issues (n=5), and vaccine hesitancy (n=3).
Conclusion: A targeted partnership with specialty pharmacy significantly improved RZV uptake among RA patients ages 18-49 years, which included direct outreach to the patients from the pharmacists. Prior to scaling the intervention to patients with various rheumatologic conditions, it is critical to investigate and address population-specific barriers to enhance vaccine uptake in this high-risk group.
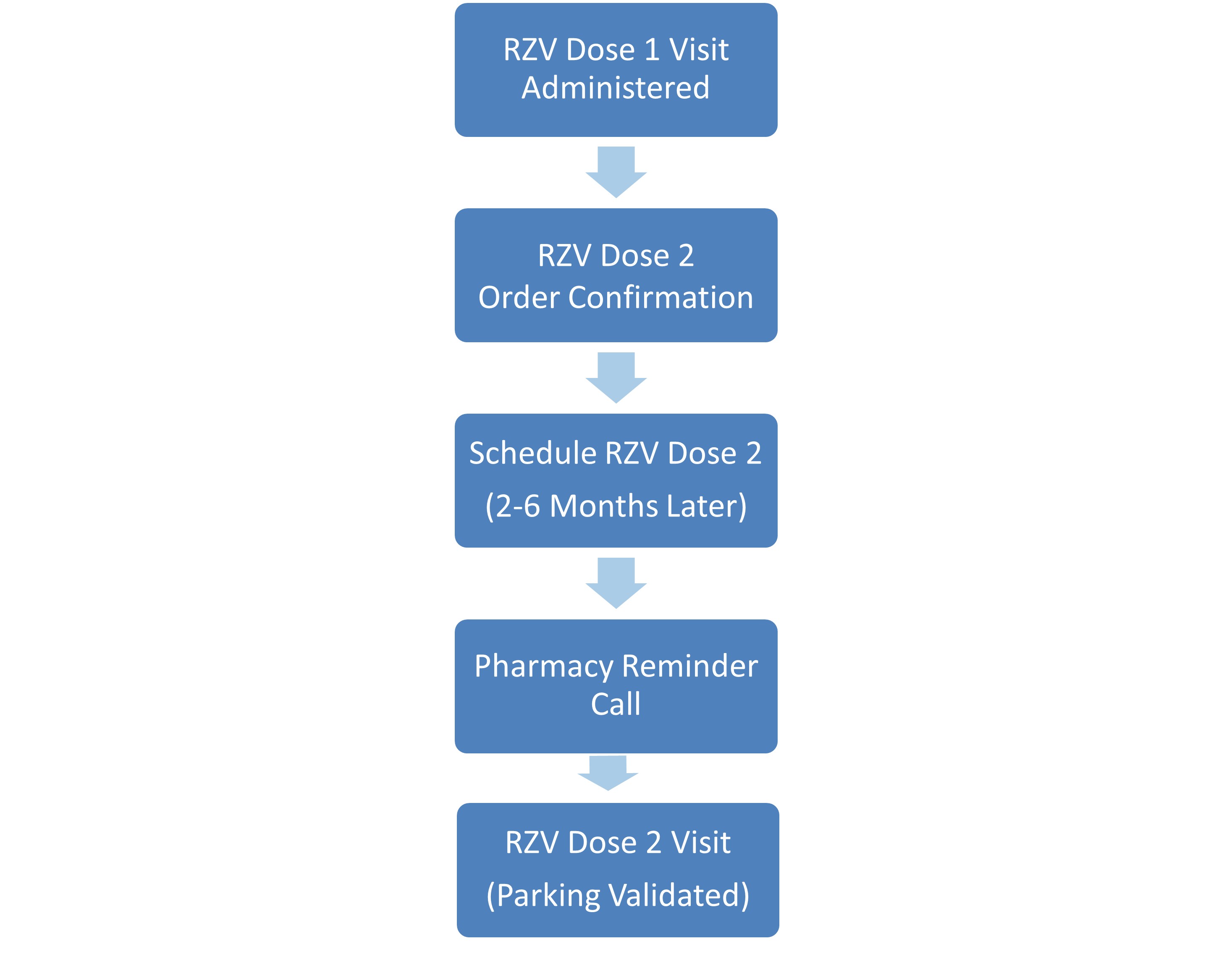 Figure 1: Process Map for RZV Dose 1 Adminstration
Figure 1: Process Map for RZV Dose 1 Adminstration
.jpg) Figure 2: Process Map for RZV Dose 2 Adminstration
Figure 2: Process Map for RZV Dose 2 Adminstration
To cite this abstract in AMA style:
Sood A, Davuluri S, Haidar S, Huang D, Ochoa A, Bill C, Lin J. Improving Recombinant Zoster Vaccine Uptake in Younger Adults with Rheumatoid Arthritis through a Partnership with Specialty Pharmacy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/improving-recombinant-zoster-vaccine-uptake-in-younger-adults-with-rheumatoid-arthritis-through-a-partnership-with-specialty-pharmacy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-recombinant-zoster-vaccine-uptake-in-younger-adults-with-rheumatoid-arthritis-through-a-partnership-with-specialty-pharmacy/
