Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Rheumatology patients are at risk for severe pneumococcal infections due to their underlying disease and immunosuppressive therapy. Current Advisory Committee on Immunization Practices guidelines recommends that immunocompromised adults aged ≥19 years receive the pneumococcal conjugate (PCV13) vaccine followed by the pneumococcal polysaccharide (PPSV23) vaccine. Our goal was to implement a nurse-driven protocol to 1) increase combined PCV13 and PPSV23 monthly vaccination rates in immunosuppressed patients aged 19-64 years old and 2) increase the overall proportion of immunosuppressed patients aged 19-64 years old who have received both PCV13 and PPSV23 vaccinations at the University of Texas Southwestern Rheumatic Diseases clinic over a two-year period.
Methods: We identified eligible adults aged 19-64 years old in the Electronic Medical Record (EMR) using a search protocol based on pre-set medication group. We obtained baseline pneumococcal vaccination rates from January 1, 2019 to December 31, 2019. We calculated the proportion of patients who were unvaccinated, partially vaccinated (received either PCV13 or PPSV23), or fully vaccinated. We developed a nurse-driven workflow for vaccination implementation in the clinic. Using Center for Disease Control (CDC) guidelines, we created a pneumococcal vaccination protocol and converted it into a university approved Standing Medical Order (SMO) to be used by the nursing staff. Post-intervention pneumococcal vaccination rates and proportions were calculated on a monthly basis and at the end of the study period. Monthly meetings were conducted to address new problems or concerns.
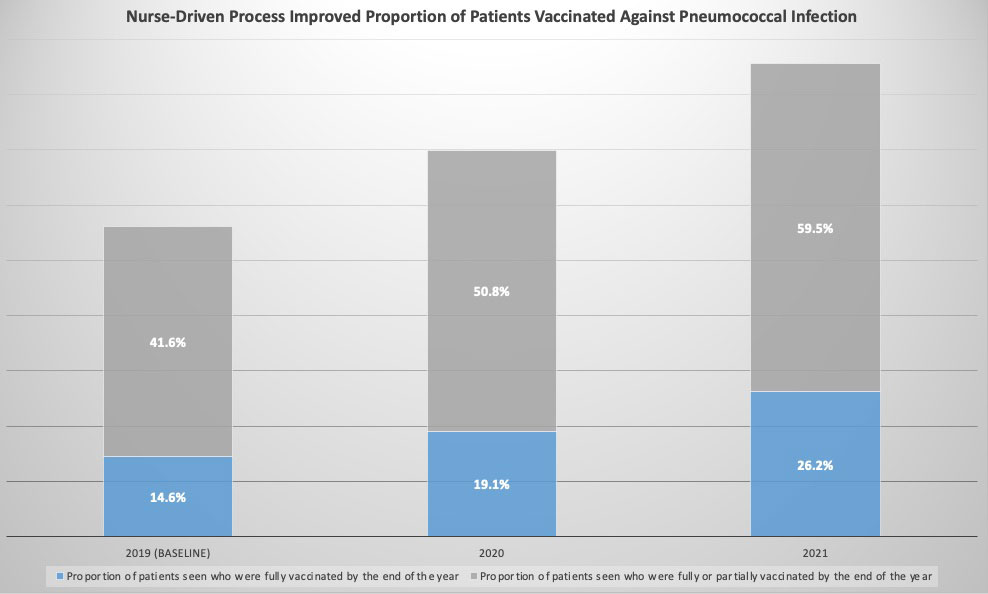
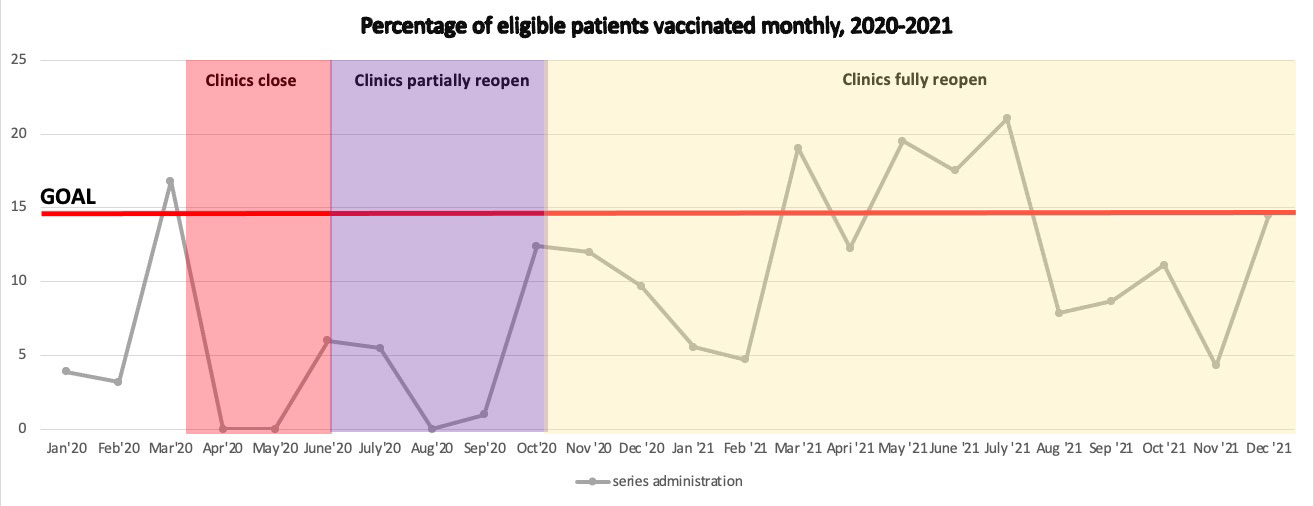
Results: The post-intervention period started on January 1, 2020. COVID-19 pandemic with associated clinic closures and workflow changes led to significant decline in vaccination rates. Vaccination rates also declined both after COVID-19 vaccines became available to patients and after the surge of COVID-19 cases due to the B.1.617.2 (Delta) variant of SARS-CoV-2. Despite that, we increased the proportion of patients who were fully vaccinated by the end of the year from baseline of 14.6% in 2019 to 19.1% in 2020 and 26.2% in 2021 (Figure 1). We also improved the rate of monthly vaccination from 6.3% to 14.5% by Dec 2021 (Figure 2).
Conclusion: Our clinic employed a nurse-driven SMO protocol that led to improvement in pneumococcal vaccination rates. The COVID-19 pandemic has provided a significant barrier to pneumococcal vaccination efforts for multiple reasons, including reduction in in-person visits, changes in clinic protocols, and providing competing priorities.
To cite this abstract in AMA style:
Joerns E, Pokala N, Wang D, Reisch J, Arasaratnam R, Bermas B, Bajaj P. Improving Pneumococcal Vaccination Rates Among Immunosuppressed Adults in an Academic Rheumatology Clinic Utilizing a Nurse Driven Protocol [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/improving-pneumococcal-vaccination-rates-among-immunosuppressed-adults-in-an-academic-rheumatology-clinic-utilizing-a-nurse-driven-protocol-2/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-pneumococcal-vaccination-rates-among-immunosuppressed-adults-in-an-academic-rheumatology-clinic-utilizing-a-nurse-driven-protocol-2/