Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Laboratory tests are routine in the management of SLE. In clinical trial endpoints, data from laboratory tests contribute to responder status, but this is captured using discrete thresholds rather than continuous data. We investigated whether the trajectory of abnormal laboratory tests in patients with active SLE, measured as continuous data, corresponds with conventional measures of clinical improvement.
Methods: We used clinical and laboratory data from a large multinational prospectively followed longitudinal cohort. Eligible patients had active disease (SLEDAI-2K ≥6), and at least one abnormal routine laboratory test (haemoglobin, white cell count, lymphocytes, platelets, ESR, albumin, C3, C4, anti-dsDNA, and urine protein, red cells or white cells), at a visit designated as baseline. At 12 months thereafter, for each laboratory test we classified improvement from individual patient baselines as complete response (CR; 100% improvement i.e. restoration to normal range), partial response (PR; ≥ 20-< 100% improvement) or no response (NR; < 20% improvement)) and assessed associations with a range of improvement outcomes: modified SLE responder index (omitting BILAG criteria) (mSRI4), physician global assessment (PGA) improvement ≥ 0.3, attainment of lupus low disease activity state (LLDAS (Golder, 2019)), SLE flare index (SFI) and SLICC/ACR damage index accrual (SDI increase >0), using logistic regression.
Results: 1,525 patients were included, with separate subsets for each individual abnormal laboratory test. Most patients were female (93%), Asian (88%) and had established SLE. Associations (odds ratios, 95% confidence intervals and significance) of laboratory variable CR and PR with clinical outcomes at 12 months are shown in Figure 1. Improvement in proteinuria, albumin, haemoglobin, ESR and platelets had the strongest associations with clinical improvement measures, including increased odds of LLDAS attainment and protection against damage accrual. In contrast, serology was associated only with the less stringent outcomes of PGA improvement and mSRI4. White cell count and lymphocytes had the weakest associations with improvement measures. Although CR had stronger associations, PR of certain laboratory tests also conferred benefit.
Conclusion: Improvements in abnormal laboratory tests were predictive of clinical outcomes at 12 months, with a discrepancy between tests currently incorporated in clinical trial endpoints, such as serology, and those with the strongest associations with clinical outcomes. Associations with improved clinical outcomes were associated with improvement thresholds less stringent than complete resolution for some tests. The selection, weighting and threshold-based use of laboratory tests in lupus trial endpoints should be revised.
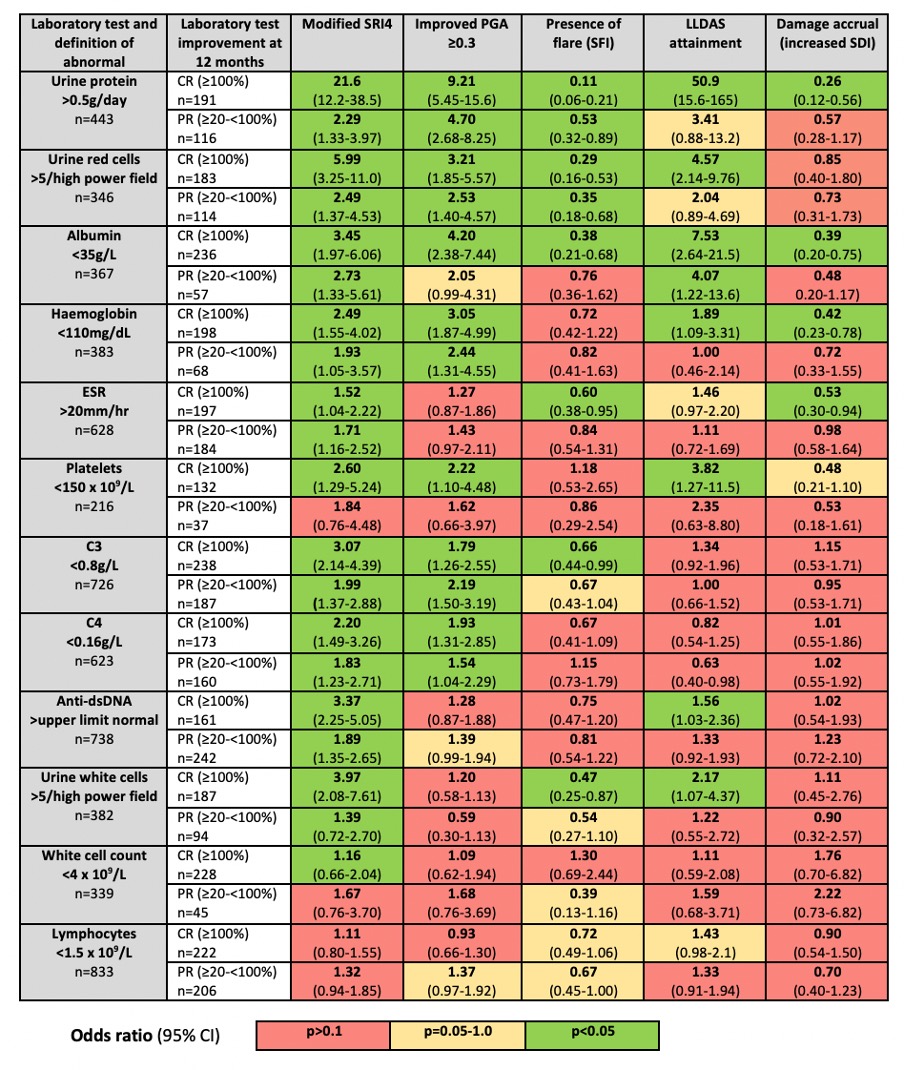 Figure 1: Associations of partial and complete improvement in abnormal laboratory tests between baseline and 12 months, and corresponding clinical improvement outcomes.
Figure 1: Associations of partial and complete improvement in abnormal laboratory tests between baseline and 12 months, and corresponding clinical improvement outcomes.
To cite this abstract in AMA style:
Connelly K, Kandane-Rathnayake R, Hoi A, Louthrenoo W, Hamijoyo L, Cho J, Lateef A, Luo S, Wu Y, Li Z, An Y, Navarra S, Zamora L, Sockalingam S, Hao Y, Zhang Z, Katsumata Y, Harigai M, Oon S, Chan M, CHEN Y, Bae S, O’Neill S, Gibson K, Goldblatt F, Kikuchi J, Takeuchi T, Ng K, Tugnet N, Basnayake B, Tanaka Y, Lau C, Nikpour M, Golder V, Morand E. Improvements in Abnormal Laboratory Tests Are Associated with Clinical Outcomes in Patients with Active Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improvements-in-abnormal-laboratory-tests-are-associated-with-clinical-outcomes-in-patients-with-active-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-abnormal-laboratory-tests-are-associated-with-clinical-outcomes-in-patients-with-active-systemic-lupus-erythematosus/
