Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: The clinical heterogeneity of the SLE makes it often challenging for the treating clinician and also remains one of the many reasons behind failed treatment trials in the past (1). Sirolimus inhibits the mechanistic target of rapamycin (mTOR), a serine-threonine kinase, which is one of the key drivers behind the pro-inflammatory immune cell lineage specification in SLE (2). We herein, aim to define the safety, tolerance and selected renal and non-renal outcome measures in SLE patients who received sirolimus therapy in our institution over the past 21 years.
Methods: We identified a total of 87 SLE patients in our institution who were on sirolimus therapy from March 2000 to March 2021 with the use of Epic EMR slicer-dicer. We included 73 patients, who met the SLE International Collaborating Clinics 2012 criteria and could tolerate sirolimus for at least 3 months (mean duration 38.8±34.9 months), and the rest were excluded. All patients received oral sirolimus with a starting dose of 2 mg daily, and further titrated based on their tolerance to maintain a therapeutic range of 6–15 ng/mL. We evaluated the influence of sirolimus on selected outcome measures. Continuous and categorical variables were analyzed by paired t-test and chi-squared test, respectively. Two-tailed p-value < 0.05 was considered significant.
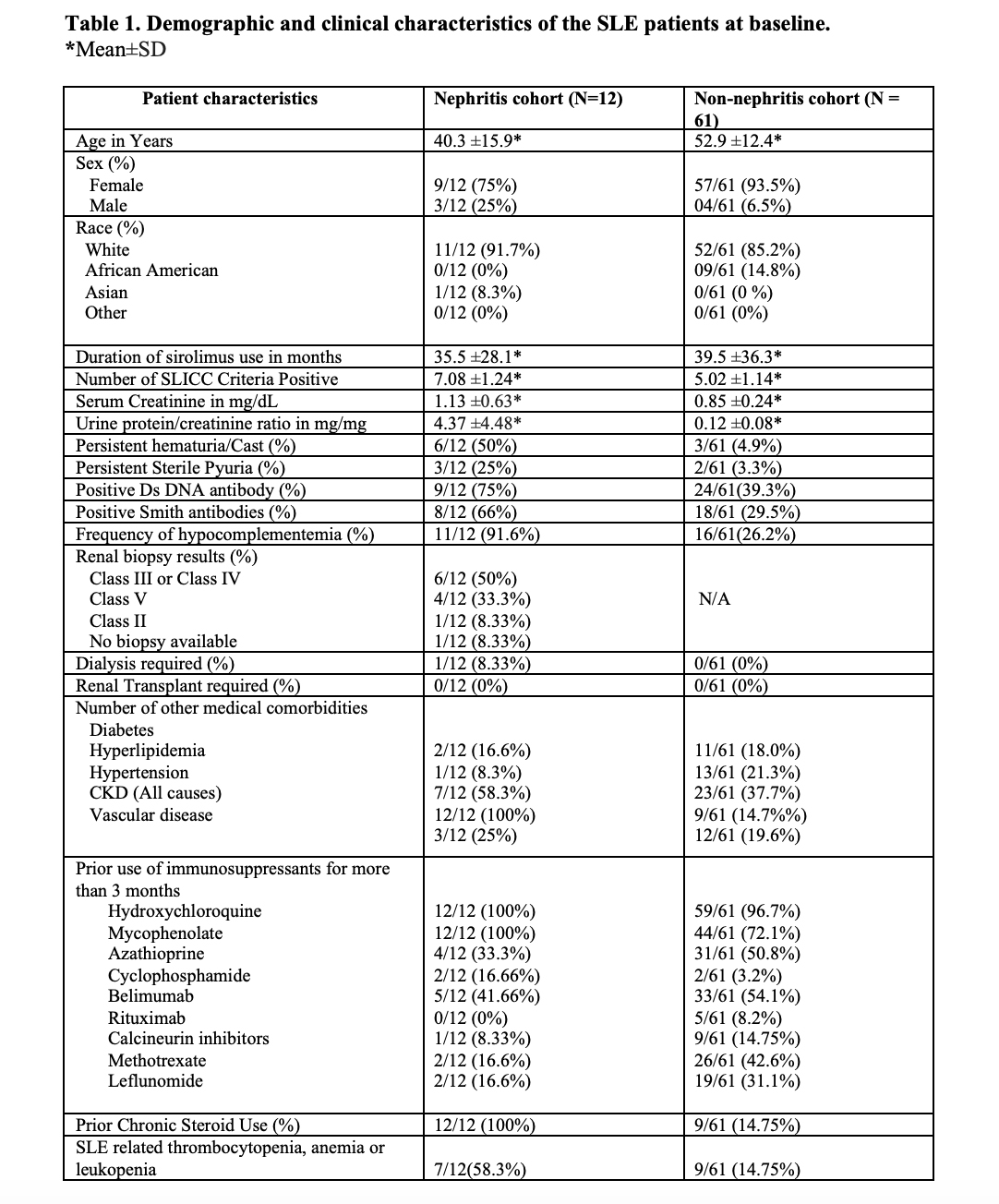
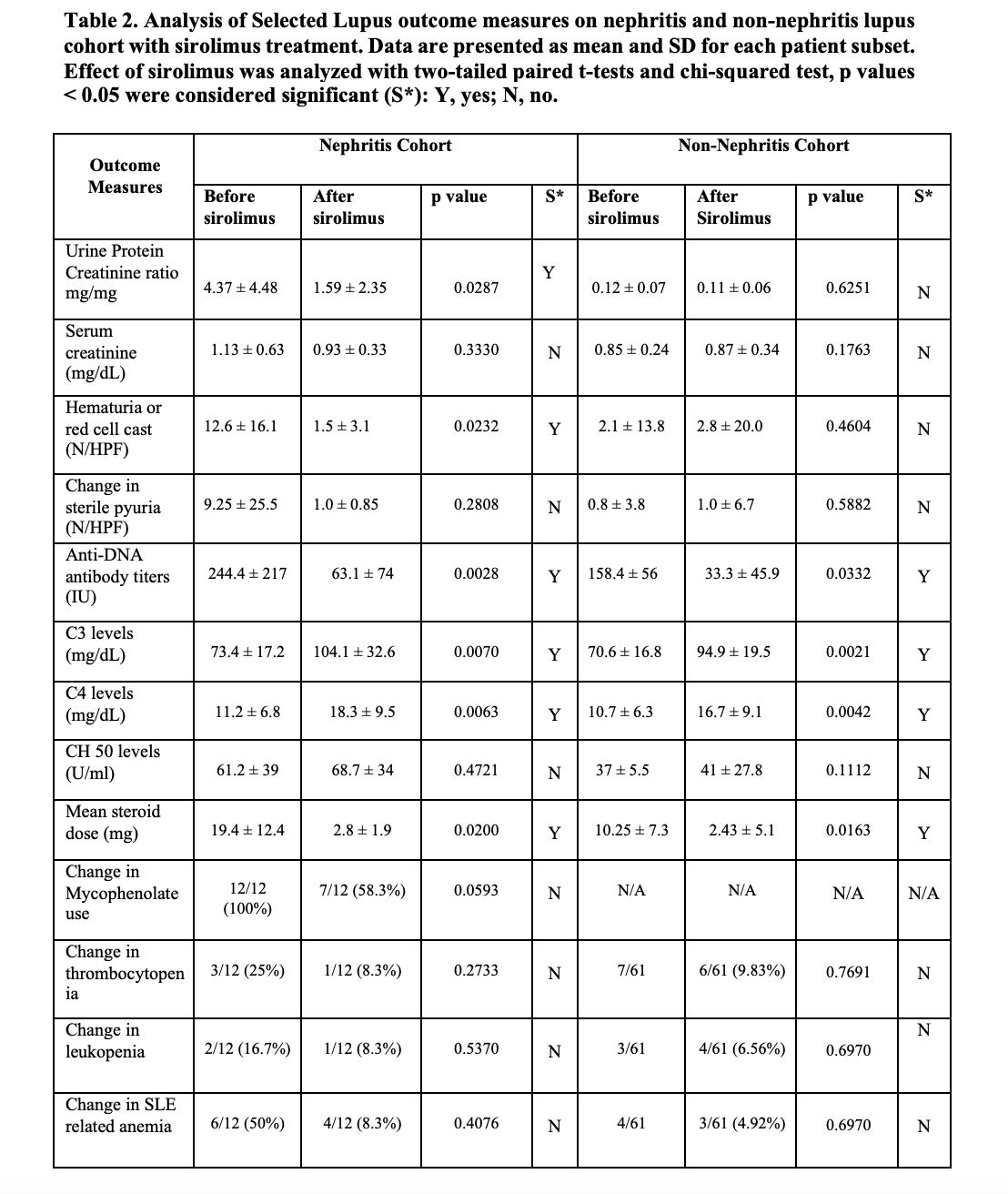
Results: The details of the baseline demographic and clinical characteristics are summarized in Table 1. The mean age of the total population is 50.6 ± 14.1, consisting of 66/73 (90.4%) females and 7/73 (9.6%) males. The ethnic diversity of the total population consists of 63/73 86.3% whites, 9 (12.3%) African Americans, and (1/73)1.4% Asians. Treatment of active lupus nephritis with sirolimus resulted in a statistically significant reduction in proteinuria (Figure 1), and microscopic hematuria. (Table 2) In the lupus nephritis cohort 12 patients, the reduction was observed in urine protein creatinine ratio (p=0.0287), hematuria (p=0.0232), anti-DNA antibody levels (p=0.0028) and mean steroid dose (p=0.0200). In the non-renal cohort of 61 patients, anti-DNA antibody levels (p=0.0332) and mean steroid dosages were reduced (p=0.0163). Both in the renal and non-renal cohorts, increases of in complement levels C3 (renal p=0.0070; non-renal p=0.0021) and C4 were observed (renal p=0.0063; non-renal p=0.0042). Adverse effects of mouth sores (2/73), headaches (1/73), and gastrointestinal discomfort were noted in a minority of patients (6/73). Sirolimus was only discontinued in two of 73 patients due to headache and recurrent infections, respectively.
Conclusion: This study suggests that sirolimus is well tolerated and exerts long-term therapeutic efficacy in controlling renal and non-renal manifestations of SLE. Double-blind placebo-controlled trials are needed to further substantiate the significance of these findings.
References
1. Francis L, Perl A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin Pharmacother. 2009 Jun 8;10(9):1481–94.
2. Perl A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Vol. 12, Nature Reviews Rheumatology. Nature Publishing Group; 2016. p. 169–82.
To cite this abstract in AMA style:
Piranavan P, Perl A. Improvement of Renal and Non-Renal SLE Outcome Measures on Sirolimus Therapy – a 21-year Follow-up Study of 73 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improvement-of-renal-and-non-renal-sle-outcome-measures-on-sirolimus-therapy-a-21-year-follow-up-study-of-73-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-of-renal-and-non-renal-sle-outcome-measures-on-sirolimus-therapy-a-21-year-follow-up-study-of-73-patients/