Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pain is a core domain of PsA, and reduction of pain is a primary treatment concern for patients with PsA. Pain has been reported to be a more important predictor of disability and poor quality of life than radiographic joint damage and disease activity. The mechanisms of pain in PsA and factors that impact the level of pain reduction in response to treatment remain elusive. In the phase 2 IM011-084 trial (NCT03881059), patients with PsA were treated with placebo or deucravacitinib 6 mg once daily (QD) or 12 mg QD. Deucravacitinib demonstrated significantly improved efficacy across multiple outcome measures compared with placebo, including improvement in Pain score (determined by visual analog scale). The aim of this post hoc analysis was to identify biomarkers associated with pain at baseline, assess the pharmacodynamic (PD) changes in these biomarkers with deucravacitinib or placebo, and better understand the relationship between baseline biomarkers and change in Pain score after treatment with deucravacitinib or placebo.
Methods: The IM011-084 trial was a randomized, double-blind, placebo-controlled, phase 2 study that enrolled 203 patients with active PsA; patients were randomized 1:1:1 to deucravacitinib 6 mg or 12 mg QD or placebo. A total of 120 biomarkers related to inflammation and extracellular matrix degradation were evaluated using ELISA, Simoa, Luminex, or Olink Target 96 Inflammation panels. Biomarkers associated with baseline global assessment of pain, PD changes, and prediction of response to deucravacitinib were analyzed.
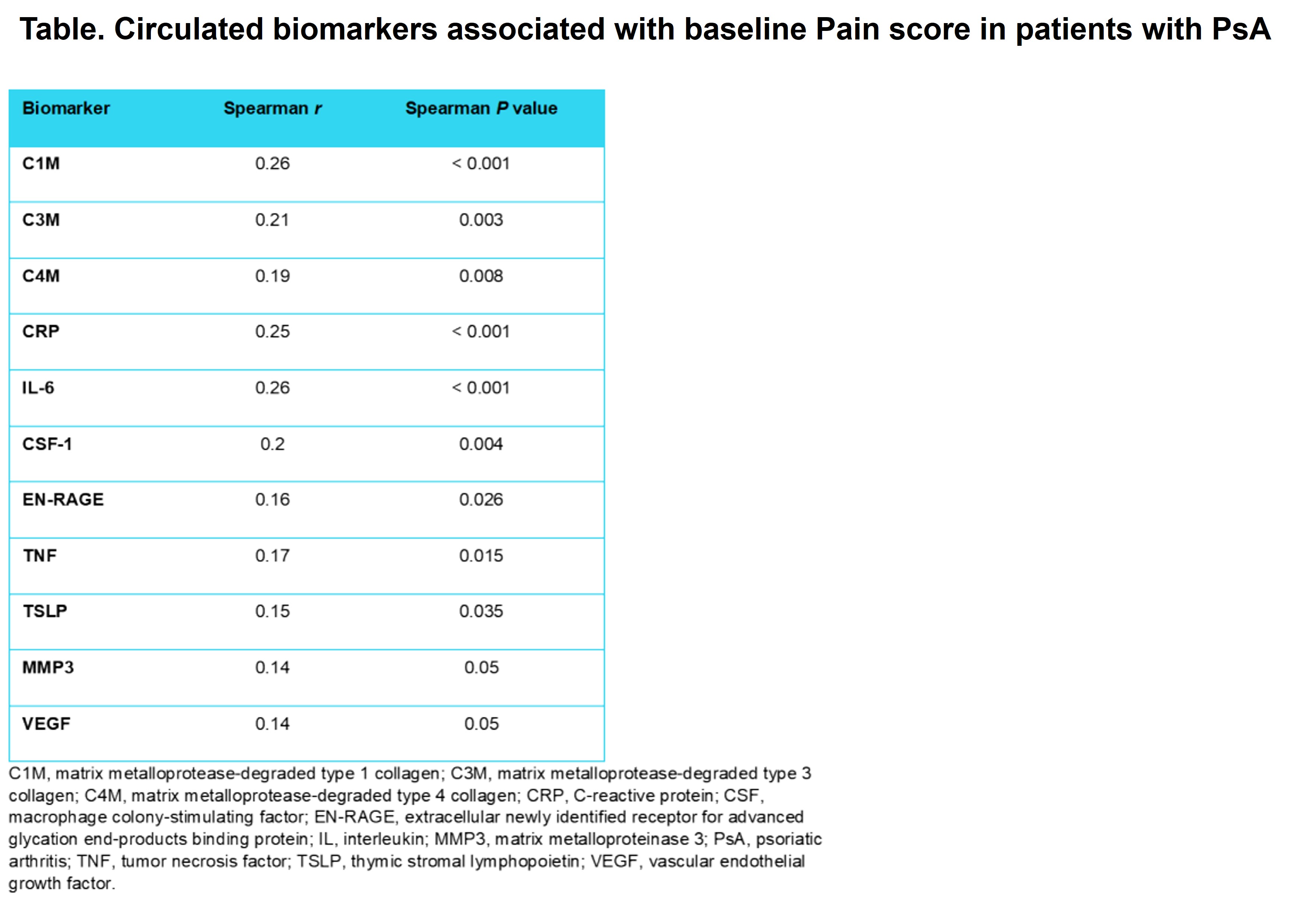
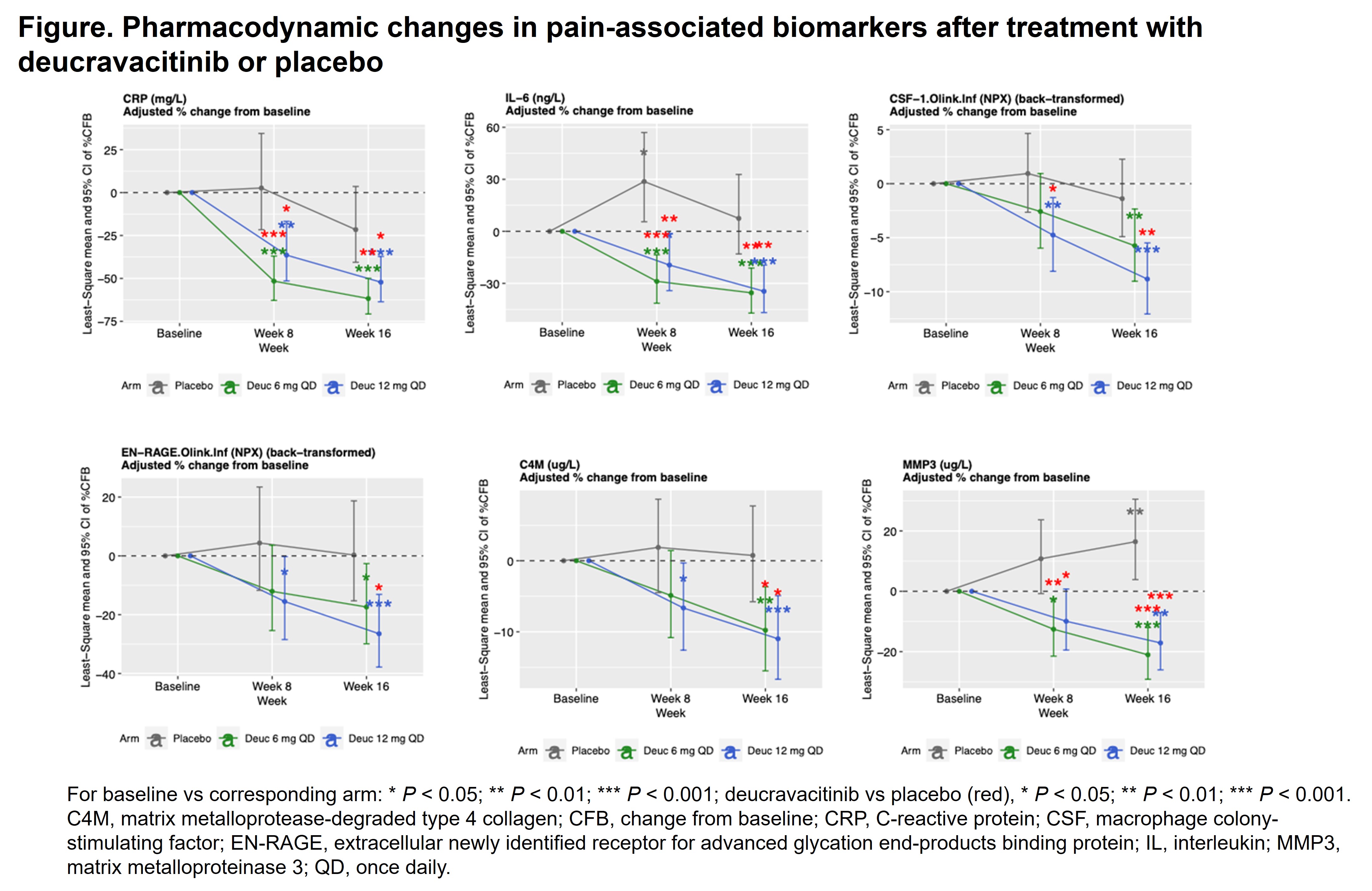
Results: Of the 120 biomarkers investigated, 11 (C1M, C3M, C4M, CRP, IL-6, CSF-1, EN-RAGE [S100A12], TNF, TSLP, MMP3, and VEGF) were significantly associated with baseline Pain score (Table). PD changes in these pain-associated biomarkers were assessed, and results from the model-adjusted analysis of percent change from baseline over the treatment course are shown in the Figure. Changes in pain-associated biomarkers in groups treated with deucravacitinib (6 mg or 12 mg) were greater than those in the placebo group. Further analysis suggested that higher baseline levels of pain-associated biomarkers (CSF-1, IL-6, and CRP) were associated with greater improvement in Pain score in patients treated with deucravacitinib but poorer response in patients treated with placebo. Patients who had greater IL-17A levels at baseline, which were associated with higher baseline Psoriasis Area and Severity Index score, had greater improvement in Pain score when treated with deucravacitinib vs placebo.
Conclusion: This post hoc analysis demonstrated that deucravacitinib reduced levels of pain-associated biomarkers vs placebo. These biomarkers are associated with peripheral and neuropathic pain. Our data suggest that deucravacitinib had a differential treatment impact on pain-associated biomarkers vs placebo; this is consistent with our findings from pain-relevant clinical outcomes. Our findings warrant further investigation into the mechanism of action of deucravacitinib in attenuating pain and the potential positive correlation between PD effects of pain-associated biomarkers and better therapeutic outcomes.
To cite this abstract in AMA style:
Mease P, Ritchlin C, Gao L, Schafer P, Nowak M, Catlett I, Liu J. Improvement in Pain-Associated Biomarkers with Deucravacitinib, a First-in-Class, Oral, Selective, Allosteric Tyrosine Kinase 2 Inhibitor, in Patients with PsA in a Double-Blind Phase 2 Study (IM011-084) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/improvement-in-pain-associated-biomarkers-with-deucravacitinib-a-first-in-class-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-patients-with-psa-in-a-double-blind-phase-2-study-im011-0/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-pain-associated-biomarkers-with-deucravacitinib-a-first-in-class-oral-selective-allosteric-tyrosine-kinase-2-inhibitor-in-patients-with-psa-in-a-double-blind-phase-2-study-im011-0/