Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: RA is more responsive to treatment in the early stages of disease, and early treatment may lead to better long-term outcomes.1,2 Data on the effectiveness of specific drugs as first or later lines of therapy will help inform treatment sequencing. Data from patients enrolled in the Corrona RA Registry network were used to compare the effectiveness of abatacept across lines of therapy overall (primary cohort) and in a subset of patients who were ACPA+.
Methods: Patients with RA who initiated abatacept (January 2006 to October 2020), had 6 months’ follow-up (within 4−9 months of starting abatacept), had baseline (BL) and follow-up CDAI scores available, and had BL CDAI > 2.8 were included. Outcomes were compared for first-, second- and third or higher-line therapy: 0, 1 or ≥ 2 prior biologic DMARDs or Janus kinase inhibitors, respectively. Continuous outcomes included change from BL to 6 months in mean CDAI and patient-reported pain, fatigue, and HAQ. Binary outcomes included rate of achieving minimal clinically important difference (MCID) in CDAI or modified ACR20/50/70 at 6 months. Continuous and binary outcomes were analyzed using multiple linear and logistic regression, respectively. The models included line of therapy, age, sex, disease duration, work status, SC nodules, history of hypertension and depression, BL CDAI, BL patient-reported pain, and BL fatigue. Additional subgroup analyses were carried out in patients with moderate/high disease activity (CDAI > 10) at BL.
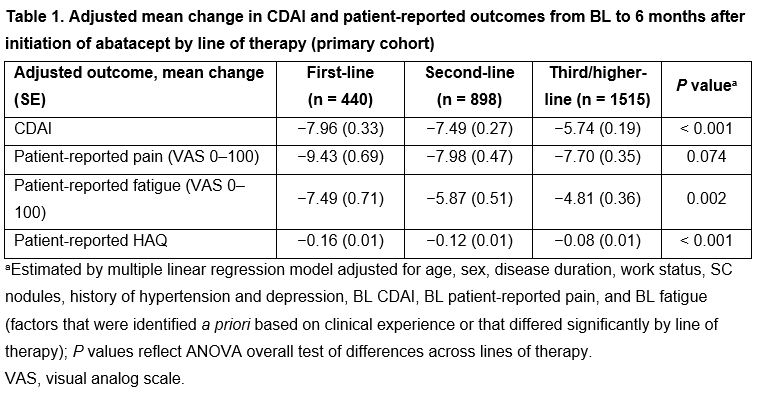
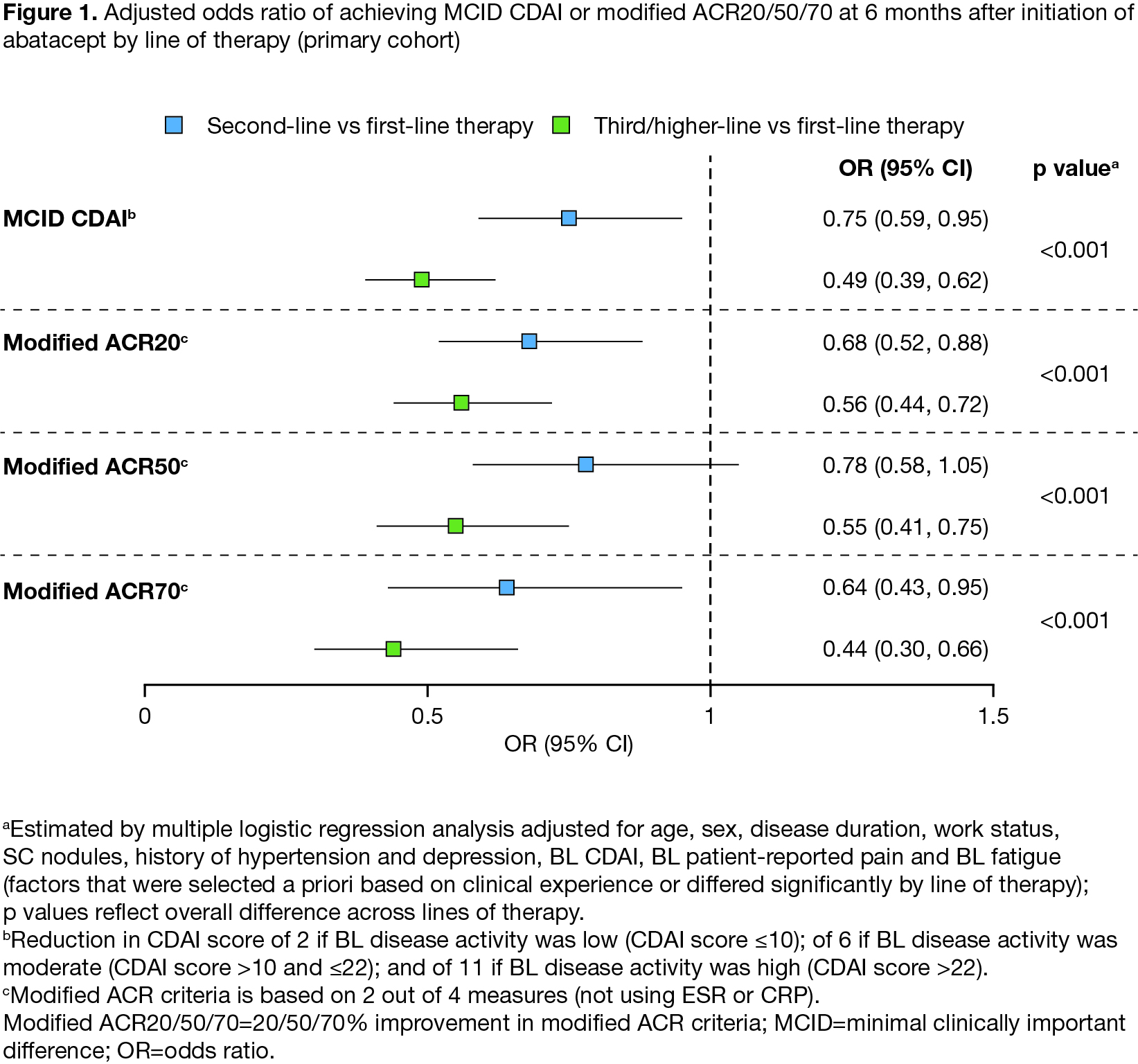
Results: In total, 2876 patients (2327 with BL CDAI > 10; 890 ACPA+) were included; 442, 911, and 1523 patients initiated first-, second- or third/higher-line abatacept, respectively. Compared with patients on second/third/higher-line abatacept therapy, those on first-line abatacept were significantly older, had shorter disease duration, and had lower BL CDAI, pain and fatigue (all P < 0.001). In adjusted analyses, patients receiving abatacept as earlier lines of therapy had significantly greater improvement from BL in mean CDAI and in patient-reported fatigue and HAQ (Table 1). There was no significant difference between lines of therapy in change in patient-reported pain. Patients receiving first-line abatacept had significantly higher odds of achieving a MCID in CDAI or modified ACR20/50/70 response (Figure 1). Similar patterns were seen when the sample was limited to patients with moderate/high disease activity or in patients who were ACPA+.
Conclusion: There were significant differences in improvement in clinical disease activity and patient-reported outcomes across lines of therapy. Better treatment responses were observed with earlier lines of abatacept therapy in the overall population, in patients who were ACPA+ and in those with moderate/high BL disease activity.
References:
1. Harrold LR, et al. Clin Rheumatol 2017;36:1215−1220.
2. Monti S, et al. RMD Open 2015;1(Suppl 1):e000057.
Medical writing: Claire Line, PhD (Caudex), funded by Bristol Myers Squibb
Original abstract © 2020 EULAR/BMJ
To cite this abstract in AMA style:
Harrold L, Wittstock K, Kelly S, Park S, Han X, Shan Y, Roberts-Toler C, Middaugh N, Khaychuk V. Improvement in Clinical Disease Activity and Patient-Reported Outcomes After 6 Months of Treatment with Abatacept, Stratified by Line of Therapy, in Patients with RA: Results from a Large, US, National Observational Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/improvement-in-clinical-disease-activity-and-patient-reported-outcomes-after-6-months-of-treatment-with-abatacept-stratified-by-line-of-therapy-in-patients-with-ra-results-from-a-large-us-nationa/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-clinical-disease-activity-and-patient-reported-outcomes-after-6-months-of-treatment-with-abatacept-stratified-by-line-of-therapy-in-patients-with-ra-results-from-a-large-us-nationa/