Session Information
Date: Monday, November 13, 2023
Title: (1221–1255) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile systemic sclerosis (jSSc) is an orphan disease with a prevalence of 3 in 1, 000, 000 children. The Juvenile Systemic Scleroderma Inception cohort (jSScC) is the largest cohort of jSSc patients in the world. The jSScC collects longitudinal data prospectively in jSSc, allowing the evaluation of the development of organ involvement and patients and physician reported outcomes in jSSc over time.
Methods: The jSScC enrolls jSSc patients who developed the first non-Raynaud´s symptom before the age of 16 years and are under the age of 18 years at the time of inclusion. We reviewed jSScC patient clinical data and patient and physician reported outcomes of those with 36 months follow up from the time of inclusion until 1stof April 2023.
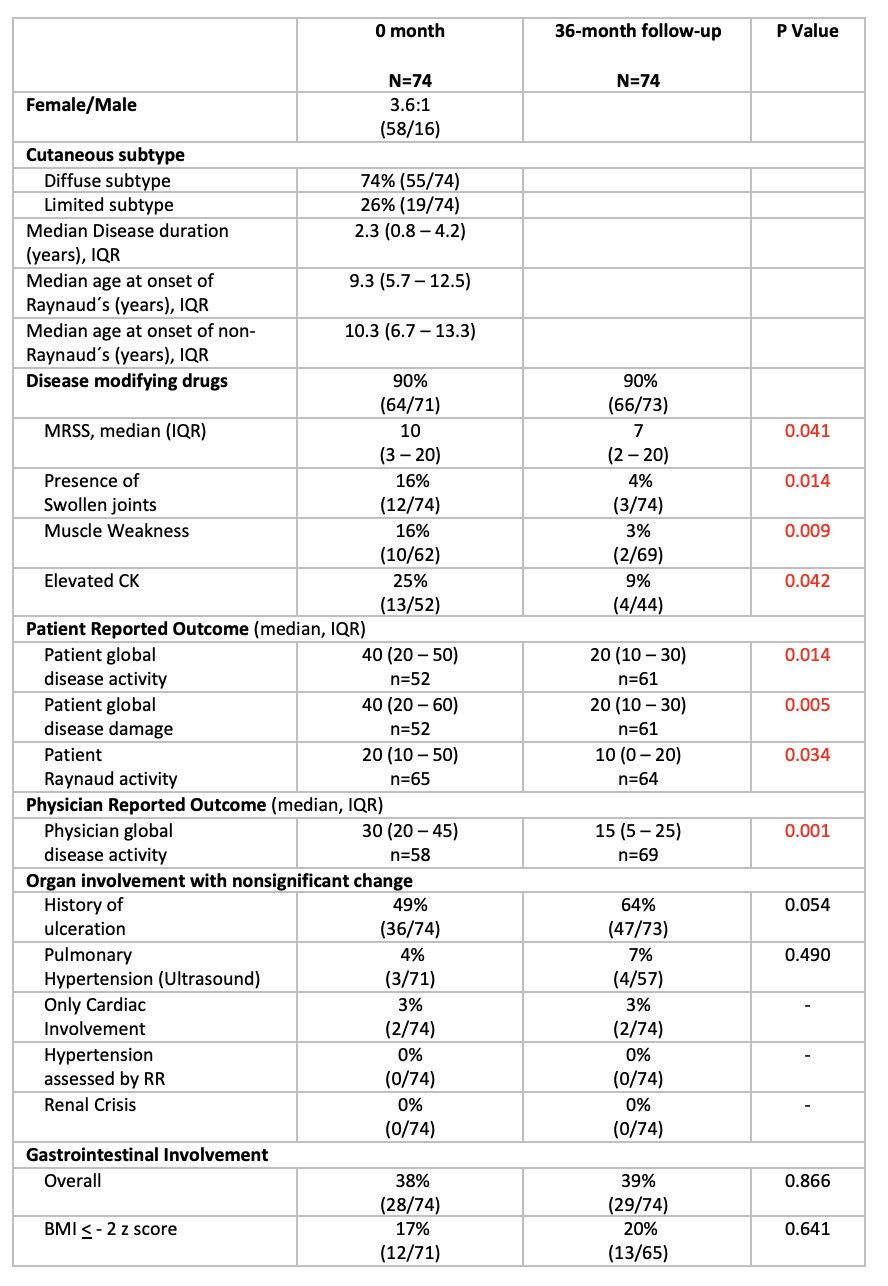
Results: We could extract data of 74 patients, 74% with diffuse cutaneous subtype. The female/male ratio was 3.6:1. 89% of the patients were Caucasian. Median age of onset of Raynaud symptom was 9.3 years and the median age of onset of non-Raynaud symptom was 10.3 years. Median disease duration was 2.3 years at the time of inclusion in the cohort (T0). Ninety percent of the patients were treated with disease modifying anti-rheumatic drugs at T0 and 90% after 36months (T36). Four clinical parameters improved significantly over time: the median modified Rodnan skin score decreased from 10 to 7 (p=0.041), the number of patients with swollen joints decreased from 16% to 4% (p=0.014), the number of patients with elevated CK value decreased from 25% to 9% (p=0.042) and the number of patients with muscle weakness decreased from 16% to 3% (p=0.009). All other organ involvement did not show any statistically significant change from T0 to T36.
Three of the four patient reported outcomes improved significantly from T0 to T36: patient reported disease activity (VAS 0 – 100) from 40 to 20 (p=0.014), patient reported disease damage (VAS 0 – 100) from 40 to 20 (p=0.005), patient reported Raynaud activity (VAS 0 – 100) from 20 to 10 (p=0.034). One of the three physician reported outcomes improved significantly: the physician global disease activity (VAS 0 – 100) from 30 to 15 (p=0.001).
Conclusion: Skin and musculoskeletal clinical features improved significantly over 36 months. It is reassuring that major internal organ manifestations, such as cardiac, pulmonary and gastrointestinal were stable. No renal crisis occurred over the 36-month time period. The patient and physician-reported outcomes had the most positive impact over the 36 months period in this large international cohort.
This project was supported by an unrestricted grant from “Joachim Herz Stiftung”
To cite this abstract in AMA style:
Foeldvari I, Klotsche J, Kasapcopur O, Adrovic A, Torok K, Feldman B, TErreri M, Sakamoto A, Anton J, Appenzeller S, Marrani E, Katsikas M, Santos M, SZTAJNBOK F, Berntson L, Brunner J, Johnson S, Kostik M, Minden K, Nuruzzaman F, Helmus N. Improvement Across Organ System, Physician and Patient Reported Outcome Measures over a 36-time Period in the Juvenile Systemic Scleroderma Inception Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/improvement-across-organ-system-physician-and-patient-reported-outcome-measures-over-a-36-time-period-in-the-juvenile-systemic-scleroderma-inception-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-across-organ-system-physician-and-patient-reported-outcome-measures-over-a-36-time-period-in-the-juvenile-systemic-scleroderma-inception-cohort/