Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: TTT has been shown in multiple RCTs to be an effective paradigm for managing RA, however TTT has not been uniformly implemented in US rheumatology. In a prior RCT, we found improvement implementation of TTT at selected rheumatology practices during a face-to-face 9-month Learning Collaborative (LC) (1). An LC is a method for group-based quality improvement, which has been successfully used in healthcare. In the current study, we tested a 6-month virtual LC that took place during the COVID pandemic as a method for improving implementation and adherence with TTT in 18 rheumatology practices in the US.
Methods: During 2020-2021, we conducted a virtual LC for TTT in RA. All meetings used video-conferencing and a website allowed participants to share documents and data. The LC consisted of one 5-6 hour kickoff session and 6 monthly webinars. Faculty and participants discussed procedures for TTT, its rationale, and methods for quality improvement; we focused on Plan-Do-Study-Act (PDSA) cycles as a method for implementing TTT. Practices were asked to meet and carry-out PDSA cycles. Each monthly webinar discussed the PDSAs, reinforced topics from the kick-off, and demonstrated data on TTT adherence from the practices. Practices were asked to review 20-25 visit notes from RA patients and to input de-identified data into the website. The data were analyzed and shared in the monthly webinars. The webinars allowed sites to discuss the data and share best (and worst) practices. Adherence with TTT was measured as a percentage of the TTT component processes: 1) measure and document RA disease activity (any standard disease activity measure considered acceptable, e.g., CDAI, RAPID3, DAS28), 2) determine a target disease activity, 3) make treatment changes if not at target, and 4) document shared decision-making.
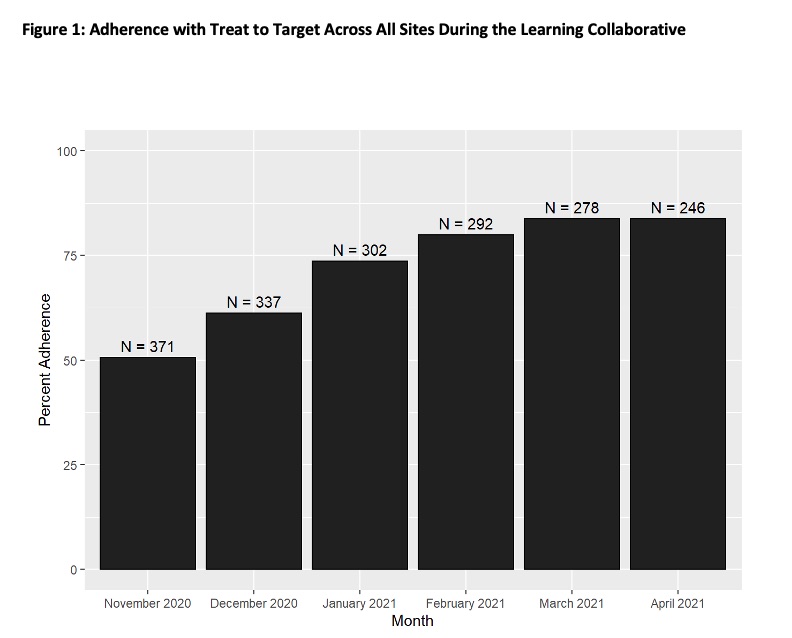
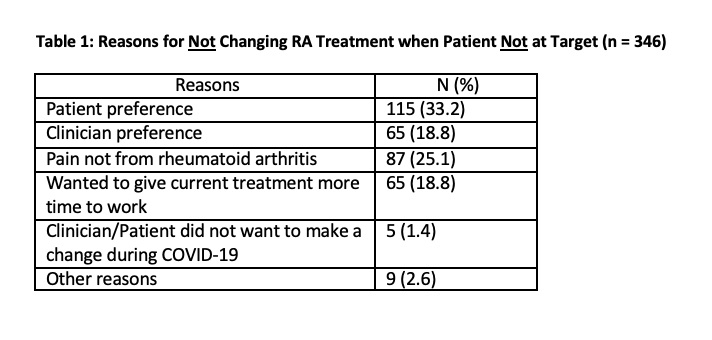
Results: 18 sites from 10 states (plus Washington DC) participated; they represented 45 rheumatology clinicians (MDs, nurse practitioners, and physician’s assistants) who attended the kick-off and/or monthly webinars. During the 6 months, sites entered data on 1826 patient visits. Adherence with TTT improved over the six months from a mean of 50.7% at baseline to 83.7% at study completion (see Figure 1, p for trend < 0.001). Each aspect of TTT measured also improved (see Figure 2). The main reason for TTT non-adherence was that clinicians changed treatment in only 59% of visits where patients were not at target. Some reasons documented (not mutually exclusive) for not intensifying treatment when patients were not at target are shown in Table 1. They include patient preference (33%), clinician preference (19%), clinician deemed symptoms were not from RA (25%), and the desire to observe the treatment effect for more time (19%).
Conclusion: Implementing TTT for RA can be improved through a relatively low-cost virtual LC. This improvement in TTT was observed despite the COVID pandemic. We would urge professional societies to experiment with implementing LC quality improvement programs virtually to facilitate wider dissemination of best practices.
1. Solomon, D. H., et al. Implementation of TTT in Rheumatoid Arthritis Through a Learning Collaborative: Results of a Randomized Controlled Trial. Arthritis Rheumatol 2017;69:1374-1380.
To cite this abstract in AMA style:
Solomon D, Pincus T, Shadick N, Stratton J, Ellrodt J, Santacroce L, Katz J, Smolen J, Chatpar P, Mundell B, Stocks M, Downey C, Torralba K, White D, Baudek M, Szlembarski S, Barnhart S, Bilal J, Lee D, Redford A, Buchfuhrer J, Kramer H, Kwoh C, Villatoro-Villar M, Patnaik A, Guzman E, Trachtman R, Tesser J, Music D, Mickey L, Amin M, Potter J, Schmukler J, Sundhar J, Sheingold J, Horowitz D, Gulko H, Quinet R, Dhulipala S, Patel R, Keshavamurthy C, Carvajal Bedoya G, Dunn R, Kumar B, Lenert A, Zembrzuska H, Gebre M, Lenert P, Anandarjah A, Yang A, Grinnell-Merrick L, Goldsmith S, Zelie J, Wise L, Zagelbaum Ward N, Kaine J. Implementing Treat to Target (TTT) for Rheumatoid Arthritis (RA) Through a Virtual Learning Collaborative (LC) Program During COVID [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/implementing-treat-to-target-ttt-for-rheumatoid-arthritis-ra-through-a-virtual-learning-collaborative-lc-program-during-covid/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementing-treat-to-target-ttt-for-rheumatoid-arthritis-ra-through-a-virtual-learning-collaborative-lc-program-during-covid/