Session Information
Date: Monday, November 14, 2022
Title: SLE – Animal Models Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: SLE is highly female-biased, yet the molecular origins of this bias remain unclear. The X chromosome contains many immune-related genes, suggesting that X-linked epigenetic dysregulation may contribute to female-biased immune responses. X-Chromosome Inactivation (XCI) is an X-chromosome-specific epigenetic regulatory mechanism that equalizes X-linked gene dosage between XX females and XY males. It is initiated and maintained by the non-coding RNA Xist, which together with various heterochromatic marks, ‘coats’ the inactive X (Xi) to effect transcriptional silencing. B and T cells uniquely exhibit dynamic XCI maintenance (dXCIm), in which naïve cells lack Xist RNA localization at the Xi despite Xist transcription. Upon cellular activation, Xist RNA and heterochromatic marks dynamically relocalize to the Xi. dXCIm is impaired in lymphocytes from the female-biased NZB/W F1 mouse model of spontaneous SLE, specifically at the late stages of disease. Using the female-biased NZM2328 and the sex-neutral MRL/lpr mouse models of spontaneous SLE, we asked whether impaired dXCIm is observed in other models of spontaneous SLE and whether it is specific to female-biased disease.
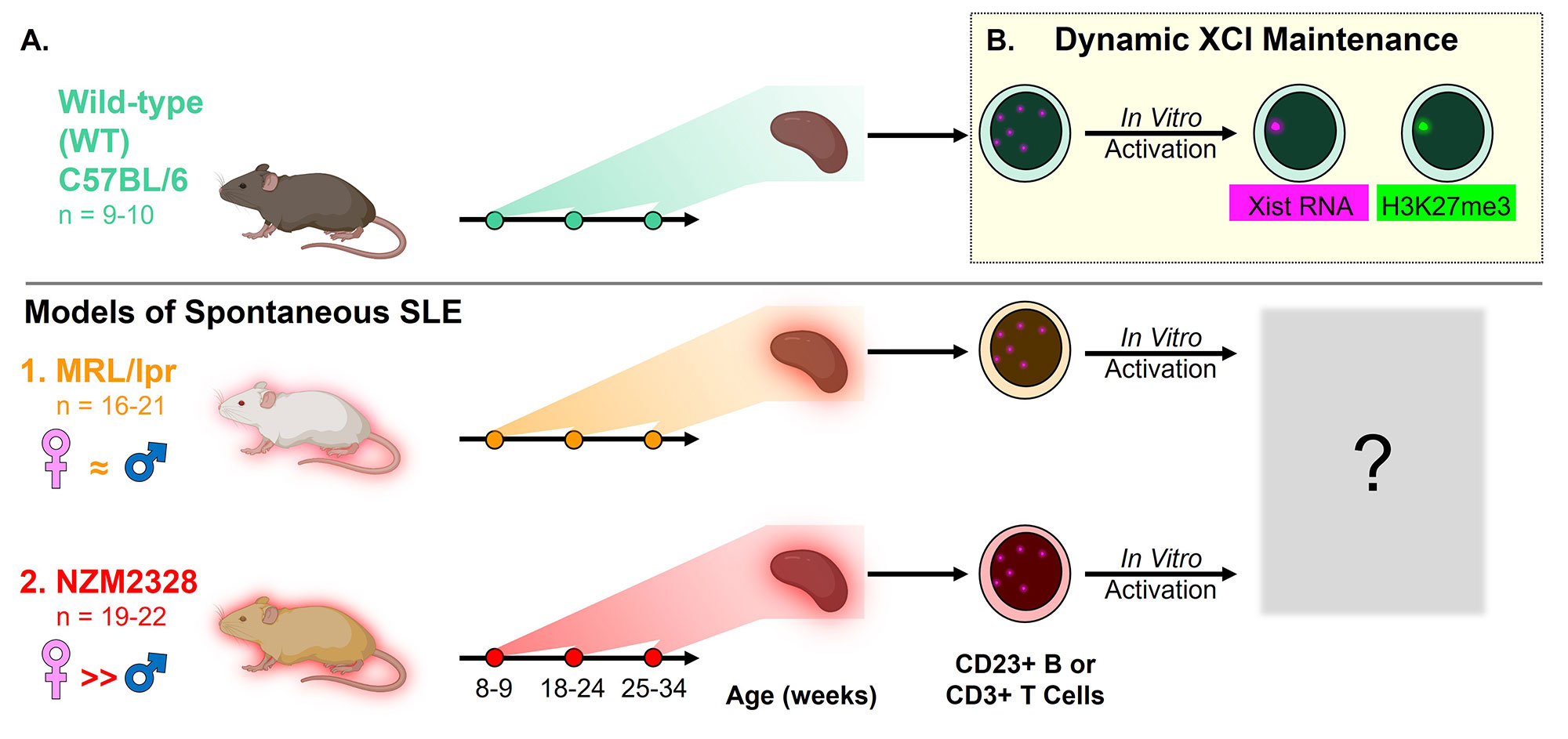
Methods: CD23+ B cells and CD3+ T cells were purified from age-matched wild-type (WT) C57BL/6, MRL/lpr, and NZM2328 female mice at three timepoints via sequential positive and negative splenocyte selection (Figure 1). B cells were activated with CpG for 24 hours and T cells with anti-CD3/CD28 for 48 hours, and both time 0 and activated cells were processed for sequential Xist RNA FISH, H3K27me3 IF, and RNAseq. Xist localization scores and H3K27me3 foci were quantified by one of two investigators based on the assessment of at least 100 nuclei per biological replicate.
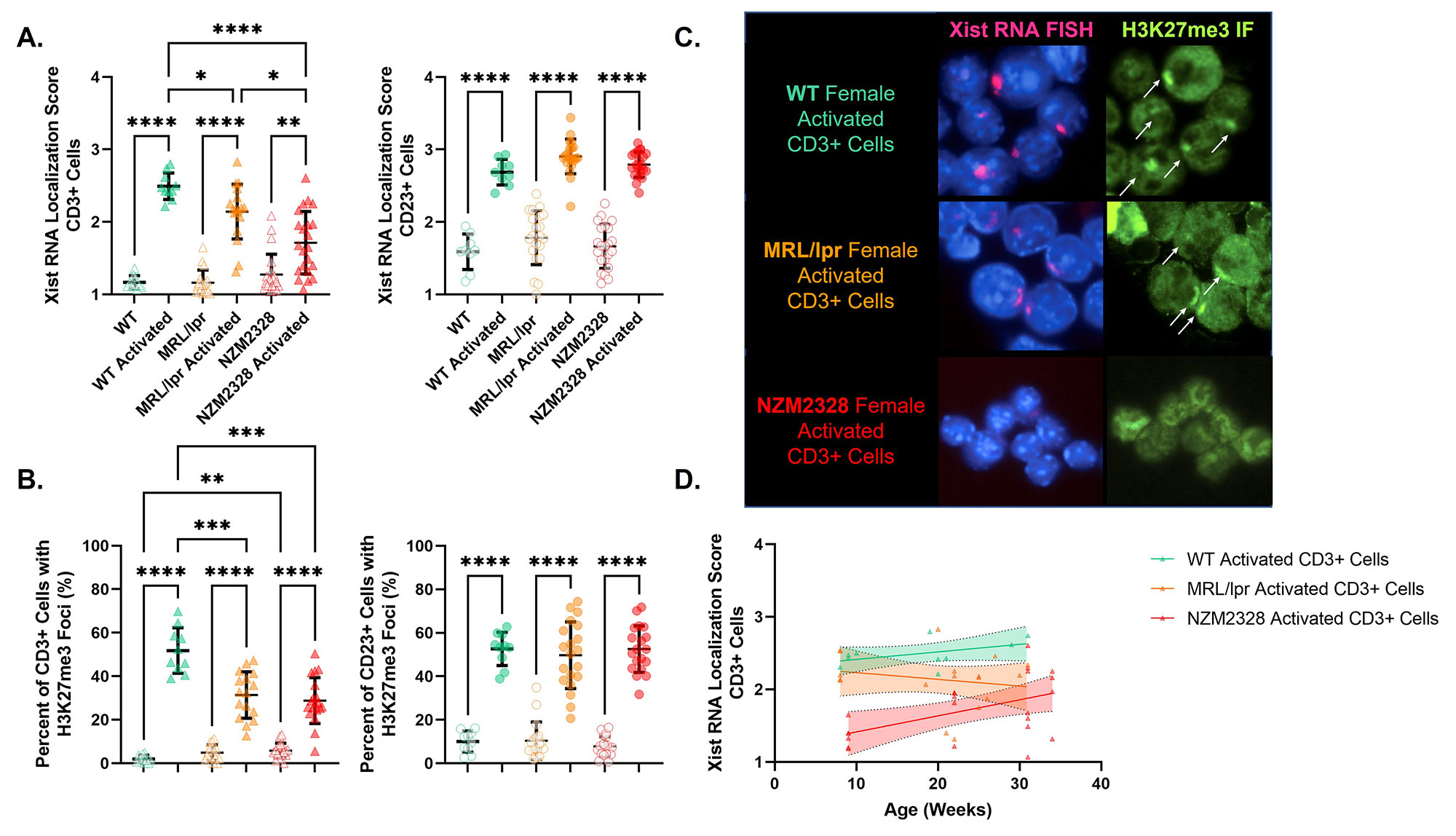
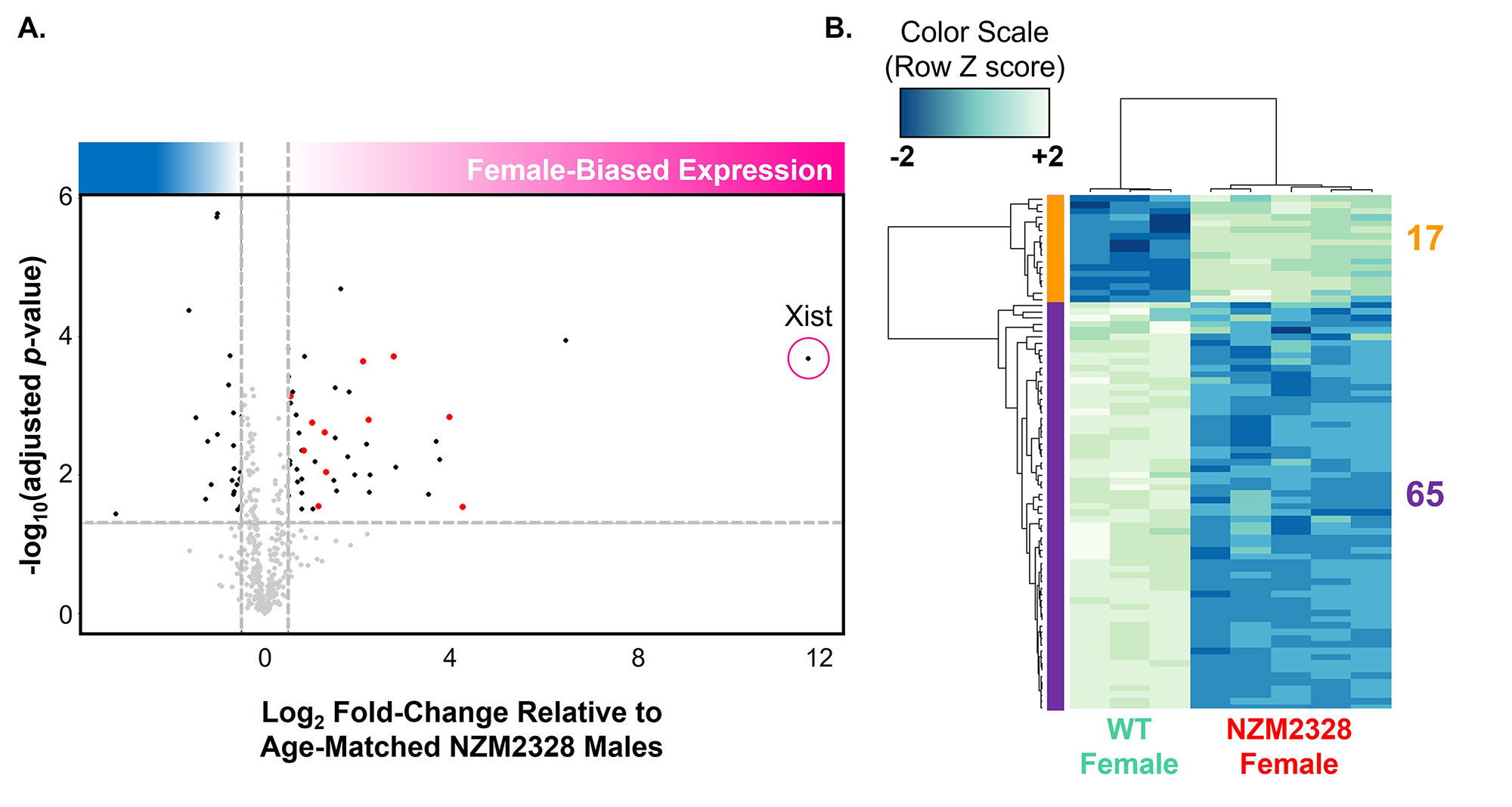
Results: Xist RNA relocalization was impaired in activated T cells from the non-female-biased MRL/lpr model (p < 0.05 vs. WT) and was markedly impaired in activated T cells from the female-biased NZM2328 model (p < 0.001 vs WT, p < 0.05 vs. MRL/lpr) (Figure 2). The percentage of T cells with H3K27me3 foci was also decreased in both MRL/lpr and NZM2328 females relative to WT female mice (p < 0.001). Conversely, Xist RNA localization and the percentage of cells with H3K27me3 foci were similar amongst activated B cells from WT, MRL/lpr, and NZM2328 female mice. While impairment of Xist RNA relocalization in T cells from MRL/lpr mice was only evident at the onset of clinical disease, impairment in NZM2328 mice was observed throughout the lifespan, before the onset of clinical disease. RNAseq of activated T cells from NZM2328 females revealed an upregulation of immune-related X-linked genes relative to male mice, as well as a significant downregulation of genes known to interact with Xist RNA (Figure 3).
Conclusion: Impaired dXCIm in lymphocytes is not specific to female-biased models of spontaneous SLE; however, the chronic, exaggerated impairment observed in T cells from the female-biased NZM2328 model suggests that abnormal dXCIm likely contributes to sex-biased disease. Given our prior findings in the female-biased NZB/W F1 model, our data suggest that abnormal dXCIm may confer female bias through diverse mechanisms involving either T cells, B cells, or both, depending on the genetic context.
To cite this abstract in AMA style:
Jiwrajka N, Toothacre N, Beethem Z, Sting S, Forsyth K, Driscoll A, Stohl W, Anguera M. Impaired Dynamic X-Chromosome Inactivation Maintenance in T Lymphocytes Is a Feature of Spontaneous Lupus in Female Mice and Is Exacerbated in Female-Biased Disease Models [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impaired-dynamic-x-chromosome-inactivation-maintenance-in-t-lymphocytes-is-a-feature-of-spontaneous-lupus-in-female-mice-and-is-exacerbated-in-female-biased-disease-models/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impaired-dynamic-x-chromosome-inactivation-maintenance-in-t-lymphocytes-is-a-feature-of-spontaneous-lupus-in-female-mice-and-is-exacerbated-in-female-biased-disease-models/