Session Information
Date: Sunday, November 12, 2023
Title: (0229–0251) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Longitudinal cohorts have described the association between gout and cardiovascular disease (CVD), while other cohort and interventional studies (CANTOS) have shown the impact of inflammatory factors on CV outcomes. Our group has demonstrated that in patients with other Rheumatic Diseases, HDL functions abnormally, promoting oxidation of LDL, rather than being anti-inflammatory. Given the gout-CVD association, we sought to evaluate the function of HDL as well as the activity of its major associated protein, paraoxonase-1 (PON1) in patients with tophaceous (TG) and non-tophaceous gout (G) compared to healthy controls (HC) and subjects with asymptomatic hyperuricemia (ASH).
Methods: Patients meeting ACR-EULAR Gout Classification Criteria were recruited from a single academic center and defined as either G or TG by presence of clinically palpable tophi. HC and subjects with ASH (serum urate > 7 mg/dL in absence of gout) were recruited.
The HDL inflammatory index (HII),is defined by the ability of patient’s HDL to prevent oxidation of LDL when added to a cell-free assay and reported as a ratio where levels > 1.0 indicate enhanced oxidation of LDL (pro-inflammatory) and ratios < 1.0 reduce oxidation of LDL (anti-inflammatory).PON1 activity was measured by paraoxanase, arylesterase and lactonase assays using paraoxon, phenylacetate, and dihydrocoumarin as substrates. Hs-CRP levels were measured by the clinical laboratory.
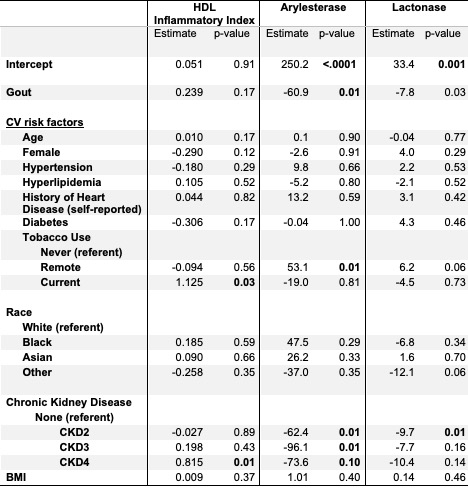
Kruskal-Wallis non-parametric analyses compared HII rank sums across clinical groups. The 5 CV biomarkers were then standardized with mean of 1 and standard deviation of 1. Generalized Linear Models compared unadjusted means across the clinical groups. Finally, multivariate regressions examined the association between gout and the 5 CV biomarkers adjusting for race, age, gender, BMI, hypertension, hyperlipidemia, diabetes, tobacco use, family history of CVD, prior heart disease and presence of kidney disease (Table 1)
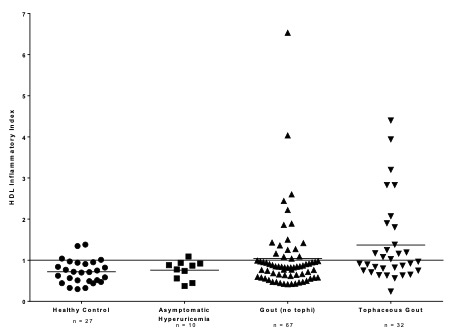
Results: Among 136 subjects, mean HII were lowest (most protective) in HC and ASH subjects (0.70 and 0.82 respectively) and highest (least protective) among G and TG patients (1.04 and 1.37 respectively), p-value = 0.0035 for the model and rank sum difference between HC and TG groupsof p < 0.05. (Figure 1)
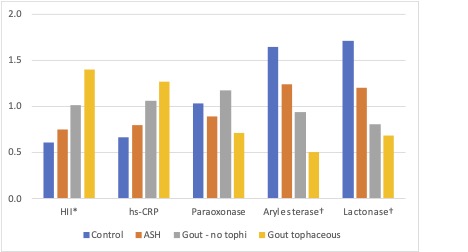
Similarly, standardized, unadjusted PON1 activity as measured by lactonase and arylesterase assays was highest (most protective) in the HC group, decreasing across the patient categories, to the lowest in the TG group (p < 0.001 for both lactonase and arylesterase differences across the groups). (Figure 2)
In multivariate analyses, PON1 activity remained most protective (arylesterase and lactonase) among HC and ASH subjects vs. G and TG patients after controlling for above covariates (p = 0.01 and 0.03, respectively). The observed gout-HII association was attenuated (p = 0.17) by the strong renal-HII association. (Table 1)
Conclusion: In this single center analysis of patients with gout (G, TG) compared to HC and patients with ASH, gout was a significant predictor of abnormal antioxidant function of HDL and decreased PON1 activity and suggests a plausible pathway for the inflammatory impact of gout, MSU crystal deposition or hyperuricemia on CVD.
Legend: HDL = High Density Lipoprotein.
P-value = 0.0035 for difference across the groups in ANOVA model.
Difference between rank sums for Healthy Controls vs. Tophaceous Gout, p-value < 0.05.
Legend: HII = High Density Lipoprotein Inflammatory Index, hs-CRP = high sensitivity c-reactive protein, ASH = Asymptomatic Hyperuricemia (urate > 7 mg/dL at time of pro-inflammatory HDL lab)
* p = 0.0165, † p < 0.001
Legend: HDL = High-Density Lipoprotein, CV = Cardiovascular, CKD = Chronic Kidney Disease
Multivariate models for gout-paraoxonase (p = 0.76) and gout-hs-CRP (p = 0.08) not shown.
To cite this abstract in AMA style:
FitzGerald J, Charles-Schoeman C, Ranganath V, Shahbazian A, Wang J, McMahon M. Impaired Anti-oxidant Function of HDL and Its Associated Protein, Paraoxonase-1, in Patients with Gout [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impaired-anti-oxidant-function-of-hdl-and-its-associated-protein-paraoxonase-1-in-patients-with-gout/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impaired-anti-oxidant-function-of-hdl-and-its-associated-protein-paraoxonase-1-in-patients-with-gout/