Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Switching treatment from conventional to biologic disease-modifying antirheumatic drugs (DMARDs) is a common practice for managing autoimmune diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PSA), and systemic lupus erythematosus (SLE). While biologics offer increased effectiveness, the impact of adherence is not clear. Investigating the influence of treatment switching is crucial for optimizing clinical and economic outcomes. The objective of this evaluation was to compare medication adherence in members that switched from conventional to biologic DMARDs to those members that did not switch.
Methods: This was a cohort study of commercial fully-insured and Medicare Advantage members of a large national health plan between 1/1/2018 and 1/1/2024. Members ≥ 18 years old were included if they had at least 2 claims for RA (ICD-10 code: M05 or M06), PSA (L40.5), or SLE (M32) during the study period. Members were excluded if they did not maintain continuous eligibility for 6 months before or after entrance into the evaluation, had multiple conditions of interest, or had missing socioeconomic status (SES) data. Members were stratified by whether they switched between conventional DMARDs and biologic medications during the evaluation period. Propensity score matching was conducted using member demographics, comorbidities, and social determinants of health (SDoH) factors. Adherence was measured using proportion of days covered (PDC), calculated as the number of days with medication available divided by the number of days elapsed between the first fill and the calculated date of exhaust of the last fill in the evaluation period. Optimal adherence was defined as PDC ≥0.8. Continuous variables were assessed with an independent t-test or Mann-Whitney U test; categorical variables were assessed with the ꭓ2 test. Logistic regression modeling was conducted between adherence and treatment switching variables; p-values < 0.05 were significant.
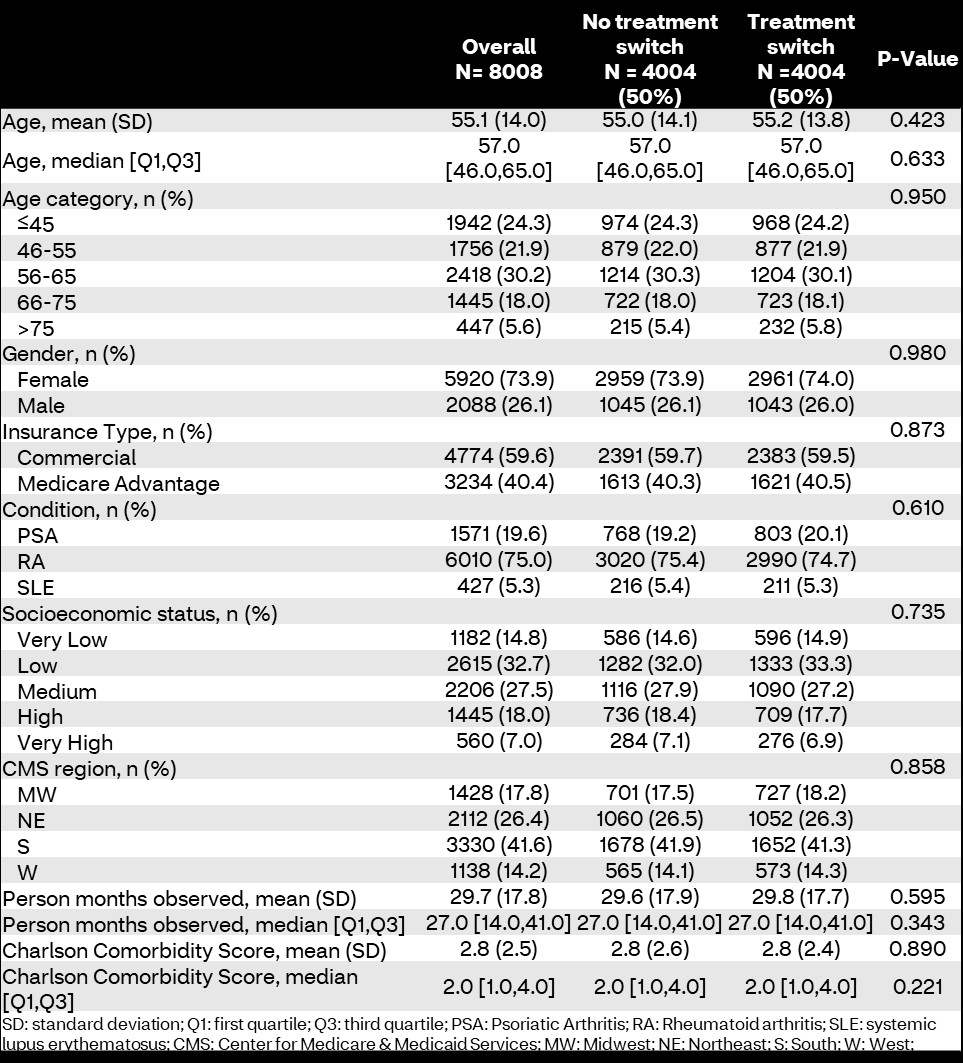
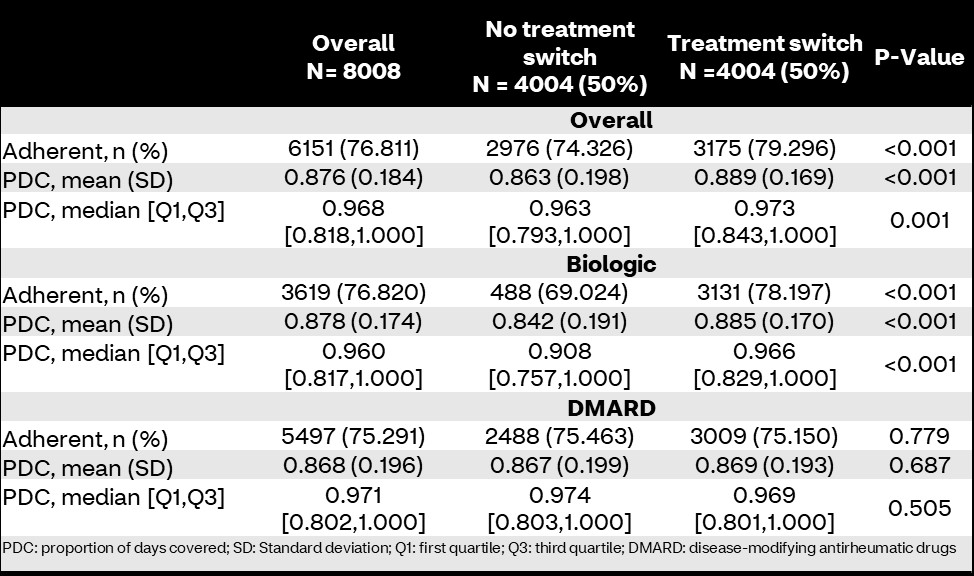
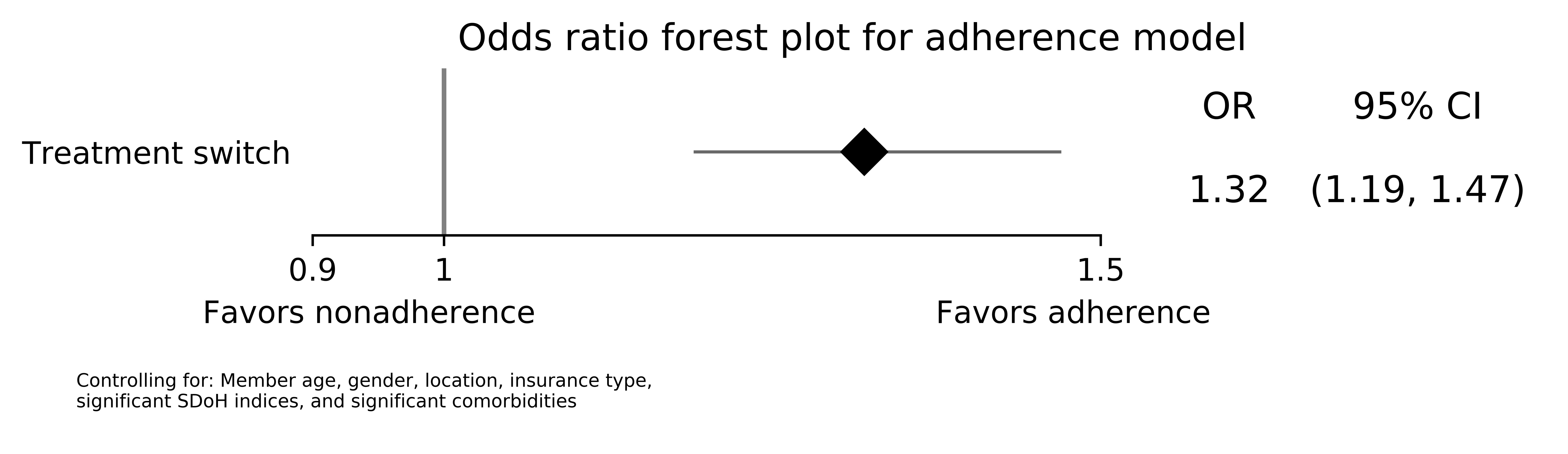
Results: 8,008 members were included and stratified by treatment switching (n=4,004; 50%). There were no differences in sociodemographic characteristics (Table 1). Overall, medication adherence was higher in members who experienced a treatment switch compared to those who did not (79.3% vs. 74.3%; p< 0.001). Additionally, PDCs were significantly higher in treatment switchers (mean PDC 88.9 [standard deviation: 16.9] vs. 86.3 [19.8]; p< 0.001). Biologic-specific adherence was higher in treatment switchers compared to those who maintained stable biologic therapy during the study (78.2% vs. 69.0%; p< 0.001); however, there were no differences in conventional DMARD adherence between groups (75.1% vs. 75.5%; p=0.779; Table 2). Treatment switching was associated with higher odds of adherence compared to non-switching in logistic regression (odds ratio [95% confidence interval]: 1.32 [1.19-1.47]; p< 0.001; Figure 1).
Conclusion: In this propensity score matched cohort study, treatment switching between conventional and biologic DMARDs was associated with increased medication adherence.
To cite this abstract in AMA style:
Park J, Rutter C, Avalos-Reyes E, Anderson M, Cavers W, Verbrugge D, Johnson K. Impact of Treatment Switching on Adherence in Members with Rheumatoid Arthritis, Psoriatic Arthritis, or Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-treatment-switching-on-adherence-in-members-with-rheumatoid-arthritis-psoriatic-arthritis-or-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-treatment-switching-on-adherence-in-members-with-rheumatoid-arthritis-psoriatic-arthritis-or-systemic-lupus-erythematosus/