Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Preclinical data suggest that tofacitinib stimulates osteoblast function and would have a protective effect on bone health and fracture risk in RA.1 We report post hoc analyses of tofacitinib clinical trials and a real-world data registry to assess the impact of tofacitinib on fracture risk in patients (pts) with RA.
Methods: We assessed fracture risk in pooled analyses of the tofacitinib RA Phase 1/2/3 and long-term extension studies (P123LTE) and the placebo-controlled portions of the Phase 3 studies, and separately in ORAL Surveillance (NCT02092467; a Phase 3b/4 randomized, open-label, post-authorization safety study, evaluating tofacitinib 5 and 10 mg twice daily [BID] vs TNF inhibitors [TNFi]).2 Incidence rates (pts with events/100 pt-years) and hazard ratios (HRs) were calculated for all fractures and osteoporotic fractures, and multivariable Cox modelling via backward selection was performed in the P123LTE cohort to identify risk factors. Data from the CorEvitas RA registry (a US-based prospective, multicenter, observational registry) were used to analyze fracture risk in pts initiating tofacitinib vs biologic (b)DMARDs. Propensity score (PS) trimming and matching was applied to the CorEvitas population to generate pt cohorts with comparable characteristics,3 and HRs were calculated for the PS trimmed and matched populations.
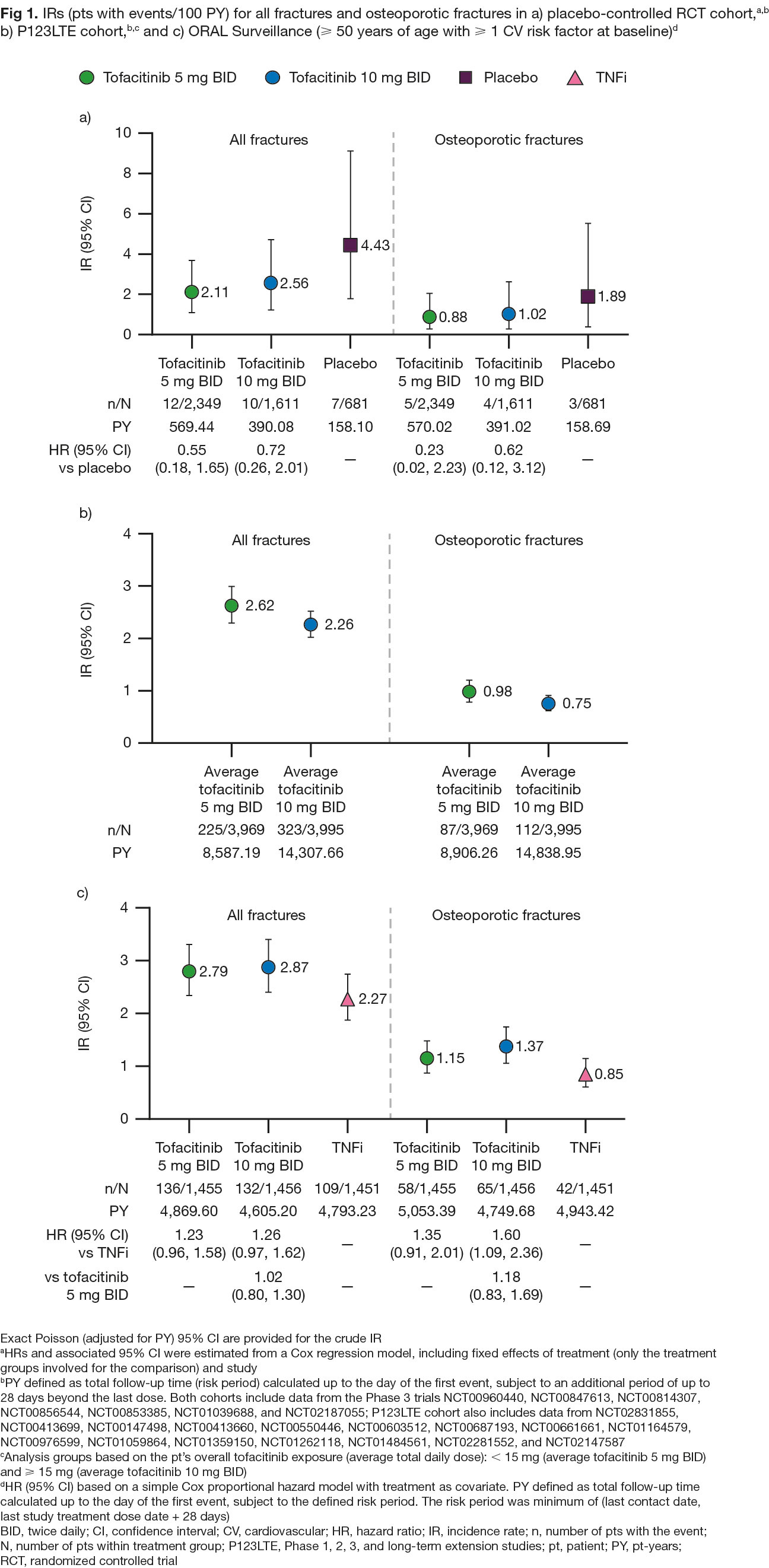
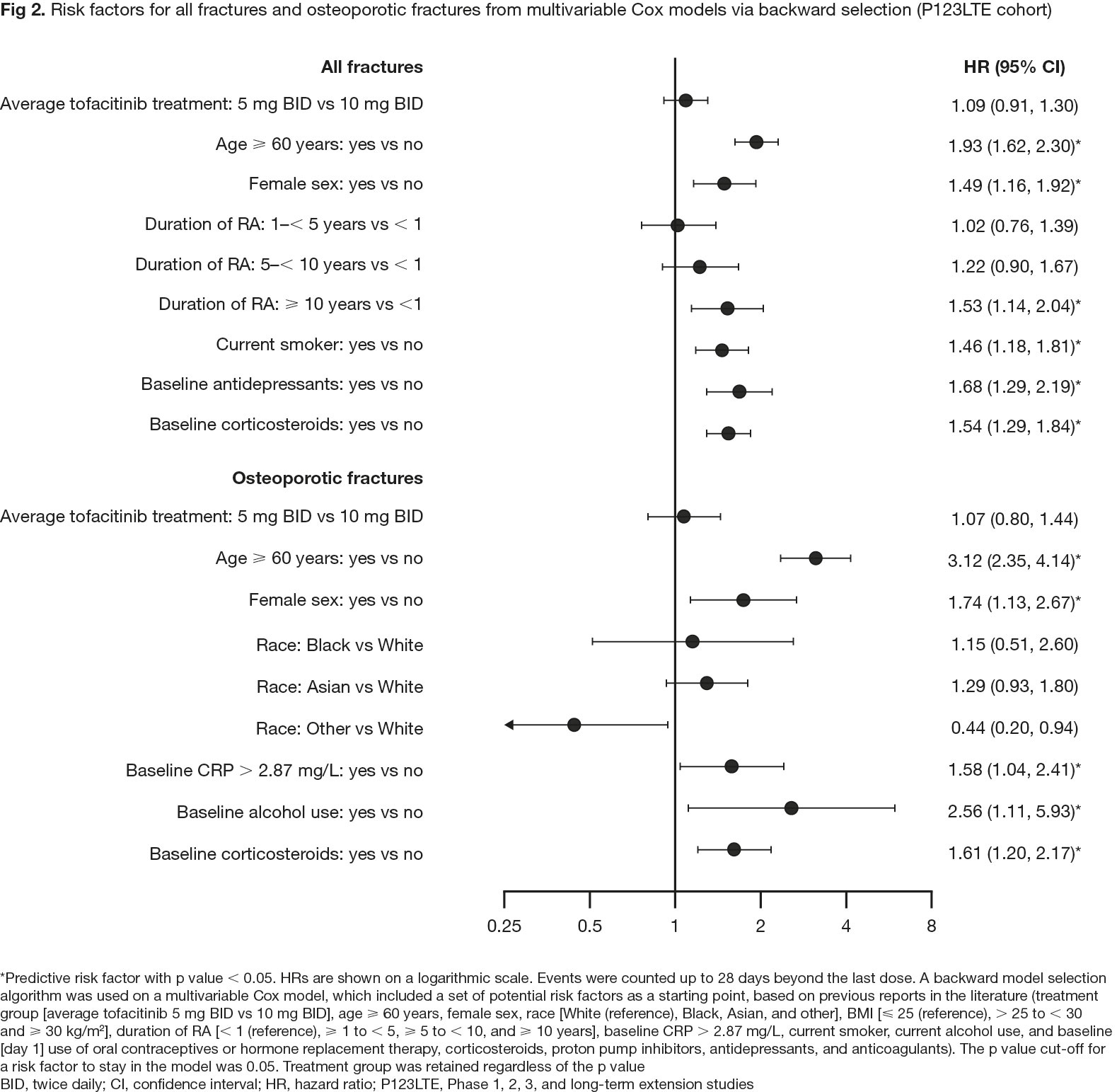
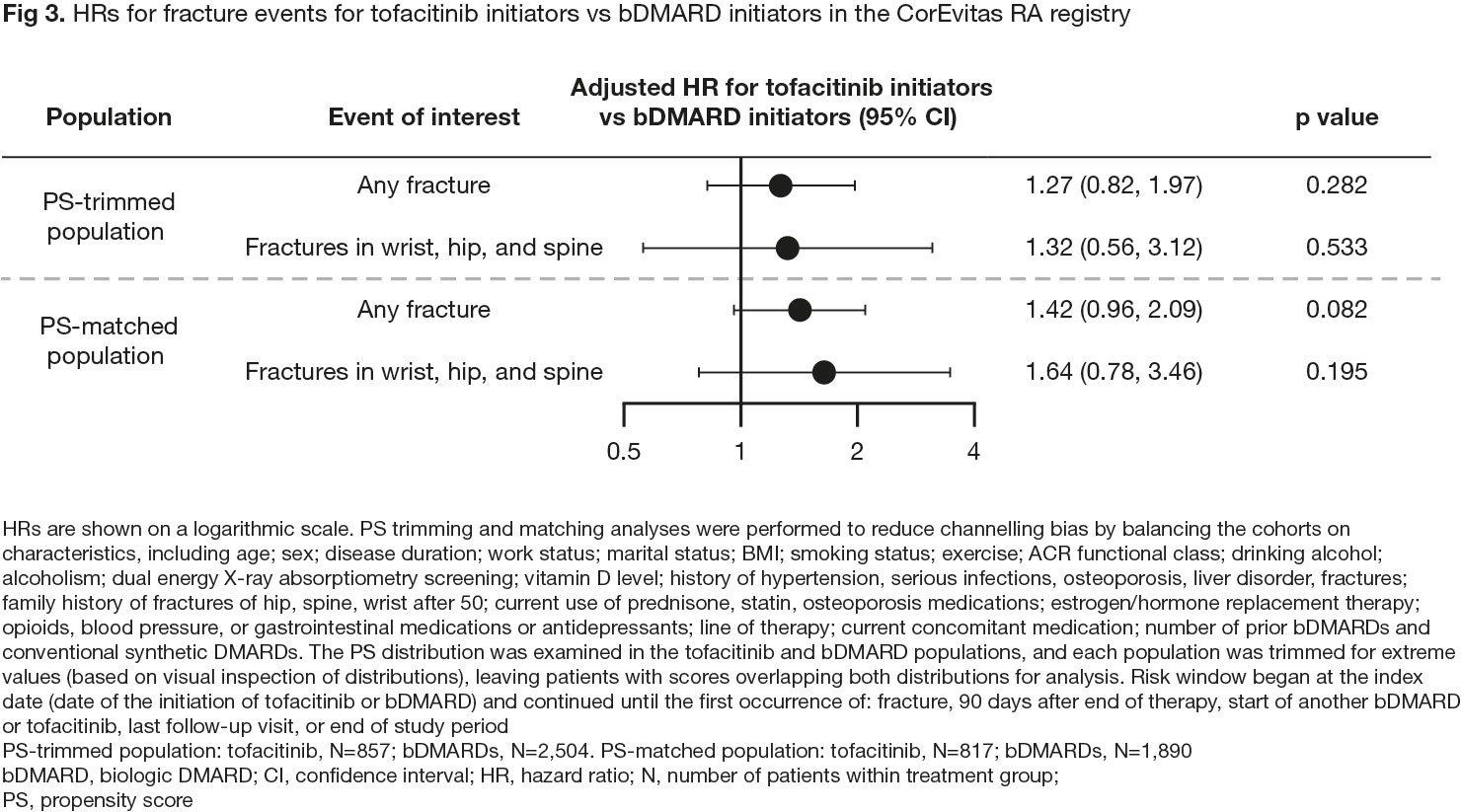
Results: In pooled clinical trial data, rates of all fractures were numerically lower with tofacitinib vs placebo (HR [95% confidence interval (CI)] 0.55 [0.18, 1.65] for 5 mg BID and 0.72 [0.26, 2.01] for 10 mg BID; Fig 1a) and were similar for tofacitinib 5 or 10 mg BID-treated pts (Fig 1a and 1b). In ORAL Surveillance, HRs (95% CIs) for all fractures with tofacitinib 5 and 10 mg BID vs TNFi were 1.23 (0.96, 1.58) and 1.26 (0.97, 1.62), respectively (Fig 1c). Risk factors for fractures in multivariable analysis included age ≥ 60 years, female sex, and baseline corticosteroid use (Fig 2), known risk factors for fractures in the general population.4 In CorEvitas registry data, HRs (95% CIs) for any fracture with tofacitinib vs bDMARDs were 1.27 (0.82, 1.97) with PS trimming, and 1.42 (0.96, 2.09) with PS matching (p > 0.05; Fig 3).
Conclusion: Pooled clinical trial data in pts with RA showed no increased risk of fractures with tofacitinib vs placebo and no clear dose effects. No unique risk factors for fractures were identified compared with those known for the general population.4 Fracture rates were numerically higher with tofacitinib vs TNFi in ORAL Surveillance. In real-world data, numerically higher fracture rates were found with tofacitinib vs bDMARDs, but the difference was not statistically significant. Pts with fracture risk factors should be monitored and treated per established standards of care.
1. Adam et al. Sci Transl Med 2020; 12: eaay4447
2. Ytterberg et al. N Engl J Med 2022; 386: 316-26
3. Kremer et al. ACR Open Rheumatol 2021; 3: 173-84
4. Kanis et al. Osteoporos Int 2008; 19: 385-97
Studies sponsored by Pfizer and CorEvitas, LLC (for the CorEvitas RA registry; analysis funded by Pfizer). CorEvitas is supported by contracted subscriptions with multiple companies. Medical writing support was provided by J King, CMC Connect, and funded by Pfizer.
To cite this abstract in AMA style:
Hansen K, Mortezavi M, Nagy E, Wang C, Connell C, Radi Z, Litman H, Adami G, Rossini M. Impact of Tofacitinib on Fracture Risk in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-tofacitinib-on-fracture-risk-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-tofacitinib-on-fracture-risk-in-rheumatoid-arthritis/