Session Information
Date: Tuesday, October 28, 2025
Title: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA is a chronic inflammatory disease affecting heterogeneous tissues, with unmet need for therapies with robust efficacy across disease domains. Sonelokimab (SLK) is a novel Nanobody that binds to both IL-17A and IL-17F with similarly high affinity. The Phase 2 ARGO trial in active PsA met the primary endpoint of ACR50 at Week (W) 12 for SLK 60mg with induction (WI) and SLK 120mg WI, with robust responses observed for high-hurdle endpoints including ACR70, PASI 100, and minimal disease activity (MDA) through W24 (McInnes et al, EULAR 2024). Patient characteristics, including sex, weight, concomitant methotrexate (MTX) use, and baseline disease activity levels may influence responses to bDMARDs (Eder et al, Lancet Rheumatol. 2023). Hence, we analyzed efficacy outcomes in ARGO according to key patient baseline characteristics.
Methods: ARGO (NCT05640245) was a global, randomized, prospective, double-blind Phase 2 trial of SLK in patients with active PsA that comprised a 12-week PBO-controlled phase followed by a further 12-week active-treatment phase. Three SLK doses were assessed: 60mg no induction (NI), SLK 60mg WI, or SLK 120mg WI (induction doses at W0, 2, 4, and 6). We report efficacy outcomes at W24 for patients assigned to the SLK arms throughout the study. Post hoc analyses were conducted to assess efficacy outcomes according to key baseline characteristics. For these analyses, data were pooled from all three SLK dose arms to maximize patient numbers.
Results: 207 patients were randomized; 49% female, 17% with weight ≥100 kg, 71% with concomitant MTX use, 17% with PASI ≥10, and 68% with DAPSA score >28 (Table). By W24, 58.1–61.0% of patients randomized to the SLK arms achieved the primary endpoint of ACR50, with robust achievement irrespective of sex, weight, concomitant MTX use, baseline PASI (≥10 vs. < 10), or DAPSA ( >28 vs. ≤28) score (Figure). The higher threshold of ACR70 was achieved by 39.0–41.9% of patients randomized to the SLK arms, with robust ACR70 responses observed across key patient subgroups, including female patients, patients with weight ≥100 kg, no concomitant MTX use, baseline PASI ≥10, and baseline DAPSA score >28 (Figure). Overall, 68.8–80.8% of patients randomized to the SLK arms achieved PASI 90 at W24, and 59.4–63.0% of patients achieved PASI 100. High levels of PASI response were observed across different subgroups, including patients with moderate-to-severe psoriasis at baseline. Overall, 46.3–61.0% of patients randomized to the SLK arms achieved MDA, with robust achievement across key subgroups, as well as in ≥40% of patients with PASI ≥10 or DAPSA score >28 at baseline (Figure). Sonelokimab was well tolerated, with a safety profile consistent with IL-17 inhibition.
Conclusion: Robust efficacy with SLK was observed irrespective of key baseline clinical characteristics in ARGO, including for populations in which disparate outcomes of treatment have been reported, such as female patients. Ongoing Phase 3 studies (IZAR-1: NCT06641076; IZAR-2: NCT06641089) will further evaluate SLK 60mg and 120mg in active PsA.
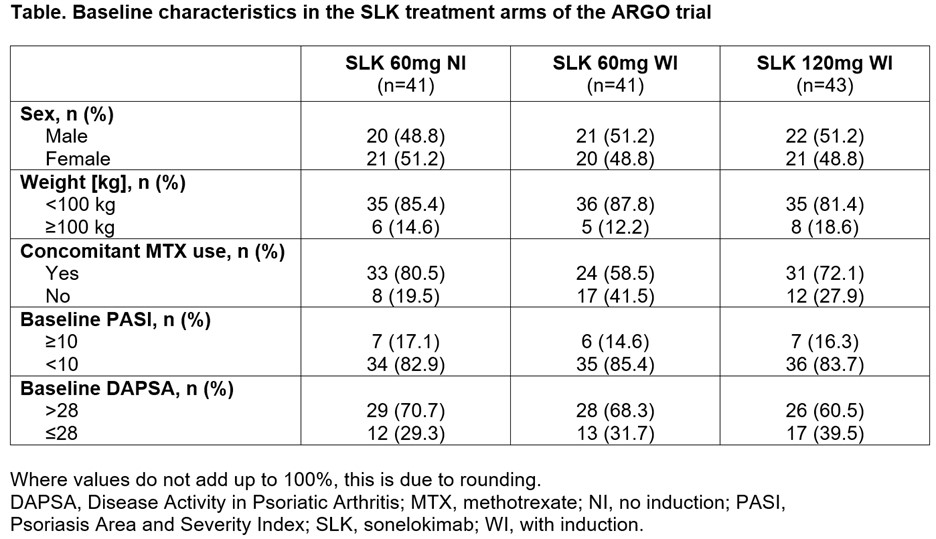 Table. Baseline characteristics in the SLK treatment arms of the ARGO trial
Table. Baseline characteristics in the SLK treatment arms of the ARGO trial
.jpg) Figure. Key outcomes at Week 24 with pooled sonelokimab doses according to clinical characteristics at baseline
Figure. Key outcomes at Week 24 with pooled sonelokimab doses according to clinical characteristics at baseline
To cite this abstract in AMA style:
Eder L, McInnes I, Ritchlin C, Ogdie A, Kavanaugh A, Coates L, Schett G, Kivitz A, Brennan N, Godwood A, Thomas M, Cullen E, Reich K, Merola J, Mease P. Impact of Sonelokimab, a Novel IL-17A- and IL-17F-Inhibiting Nanobody, in Active Psoriatic Arthritis: Key Subgroup Analyses in the Randomized, Double-Blind, Placebo-Controlled Phase 2 ARGO Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-sonelokimab-a-novel-il-17a-and-il-17f-inhibiting-nanobody-in-active-psoriatic-arthritis-key-subgroup-analyses-in-the-randomized-double-blind-placebo-controlled-phase-2-argo-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-sonelokimab-a-novel-il-17a-and-il-17f-inhibiting-nanobody-in-active-psoriatic-arthritis-key-subgroup-analyses-in-the-randomized-double-blind-placebo-controlled-phase-2-argo-trial/
