Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Sodium-glucose cotransporter-2 inhibitors (SGLT2is), initially approved for type 2 diabetes (T2D), have been demonstrated to reduce serum urate levels [1-4] and are associated with lower risk of incident gout [5-9] and recurrent gout flares [10-11] compared to other second-line glucose-lowering agents. This has raised the question of whether SGLT2i should be integrated into the treatment regimen of patients with gout. The aim of the present study was to examine whether patients with gout who initiated SGLT2i or glucagon-like peptide-1 receptor agonist (GLP1-RA) had different rates of gout-related medication and healthcare utilization thereafter.
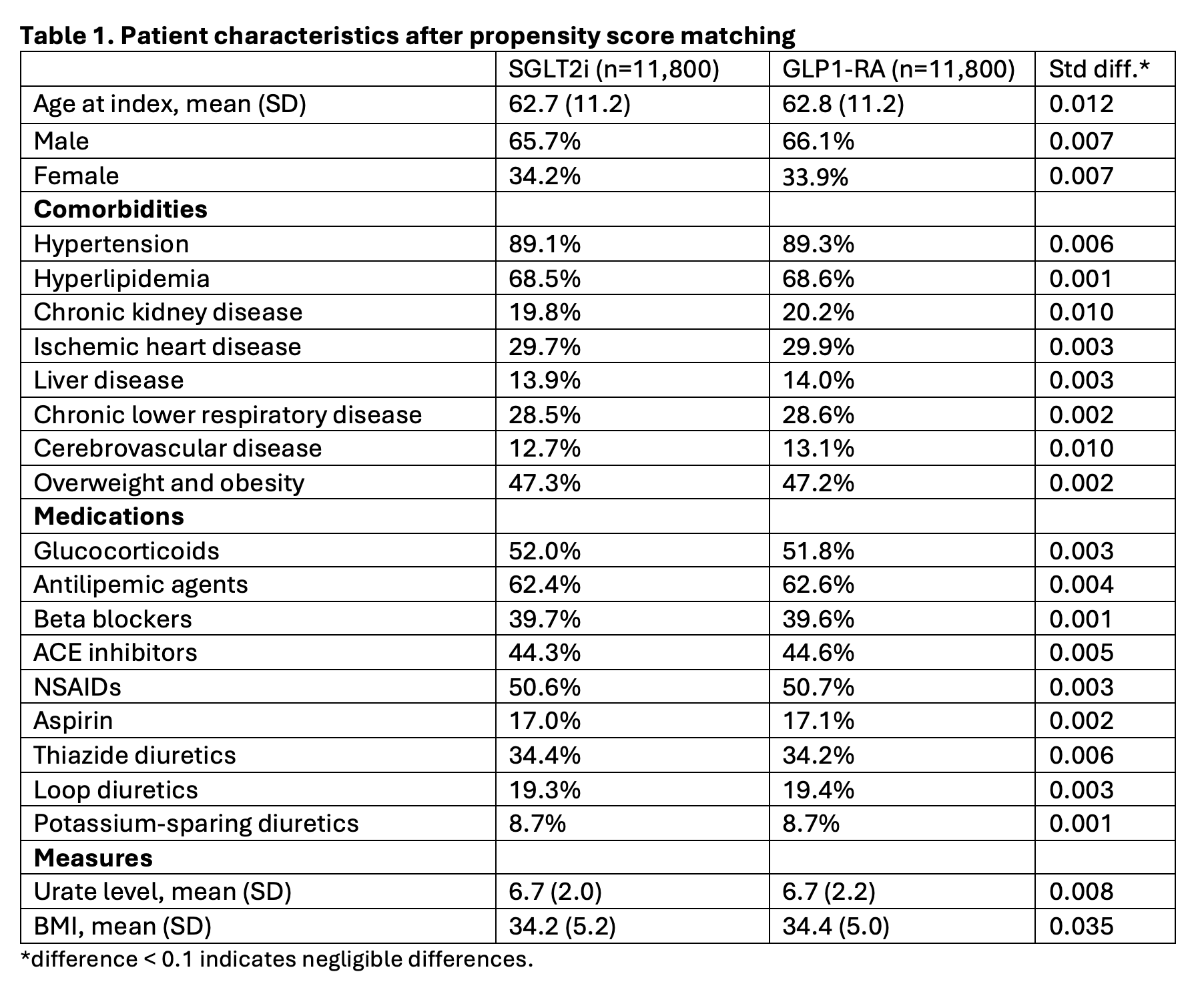
Methods: We conducted a cohort study using the TriNetX Diamond network, a large, multicenter network of U.S. claims data. International Classification of Diseases, Tenth Revision (ICD-10) codes identified patients with gout who were not on urate-lowering therapy. All patients had been diagnosed with both T2D and gout prior to initiation of SGLT2i or GLP1-RA. We analyzed the rate of initiation of urate-lowering therapy and colchicine (gout-specific flare or prophylaxis mediation) in addition to rate of gout-primary medical visit/encounter at 5 years after patients started therapy with either SGLT2i or GLP1-RA. Propensity score matching included 112 factors to adjust for demographics, comorbidities, medications, and serum urate level; some representative covariates are included in Table 1. Kaplan-Meier analysis and Cox proportional hazard models estimated the risk of the outcomes of interest.
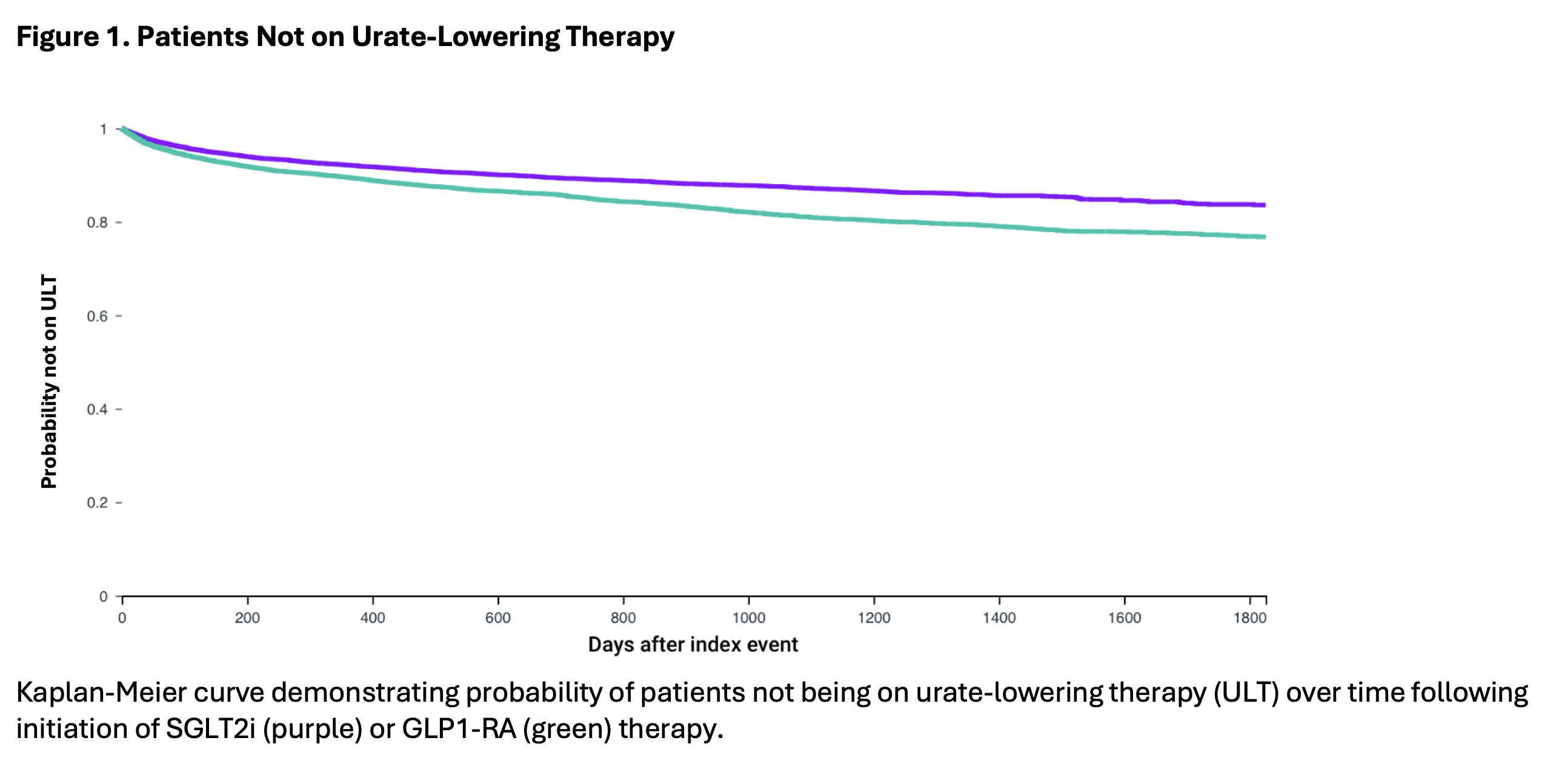
Results: A total of 16,046 patients started on SGLT2i and 16,104 patients started on GLP1-RA therapy were included. After 1:1 propensity score matching, 11,800 patients in each group were compared with well-balanced baseline characteristics (Table 1; all standardized mean differences < 0.1). Initiation of SGLT2i was associated with a lower risk of initiating urate-lowering therapy with hazard ratio (HR) of 0.69 (95% CI: 0.64, 0.75). SGLT2i was also associated with a lower risk of colchicine prescription (HR of 0.82 with 95% CI: 0.75, 0.89). HR for gout-primary medical visit/encounter was also lower (HR of 0.94 with 95% CI: 0.90, 0.99) among SGLT2i initiators.
Conclusion: This large population-based study of gout patients with T2D suggests that the urate-lowering benefit resulting from initiation of SGLT2i therapy has reduced the need for initiation of urate-lowering therapy and flare therapies. This provides further support for the use of SGLT2i therapy in patients with gout, particularly those with high-risk multi-morbidity and polypharmacy.
References
1. PMID: 28846182
2. PMID: 34617381
3. PMID: 31485187
4. PMID: 35342538
5. PMID: 31931526
6. PMID: 34797368
7. PMID: 36066415
8. PMID: 25600248
9. PMID: 33881179
10. PMID: 37487215
11. PMID: 37624597
To cite this abstract in AMA style:
Challener G, sheng-kai ma k, Kohler M, Yokose C, Yinh J, McCormick N, Choi H. Impact of SGLT2i Initiation on the Need for Urate-Lowering Therapy and Colchicine Among Gout Patients with Type 2 Diabetes:Propensity-Score Matched, Active Comparator, New User Design Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-sglt2i-initiation-on-the-need-for-urate-lowering-therapy-and-colchicine-among-gout-patients-with-type-2-diabetespropensity-score-matched-active-comparator-new-user-design-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-sglt2i-initiation-on-the-need-for-urate-lowering-therapy-and-colchicine-among-gout-patients-with-type-2-diabetespropensity-score-matched-active-comparator-new-user-design-study/