Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: In patients with RA who had a prior inadequate response or intolerance to biologic DMARDs, the oral Janus kinase inhibitor, upadacitinib (UPA), demonstrated superiority in change from baseline in DAS28(CRP) and DAS28(CRP)< 2.6 at week 12 and improved responses across additional endpoints compared to abatacept (ABA) in the phase 3 SELECT-CHOICE study.1 Seropositive patients have been reported to respond better to treatment than seronegative patients;2 therefore, the objective of this sub-group analysis was to evaluate clinical responses with UPA versus ABA among RA patients based on serologic status.
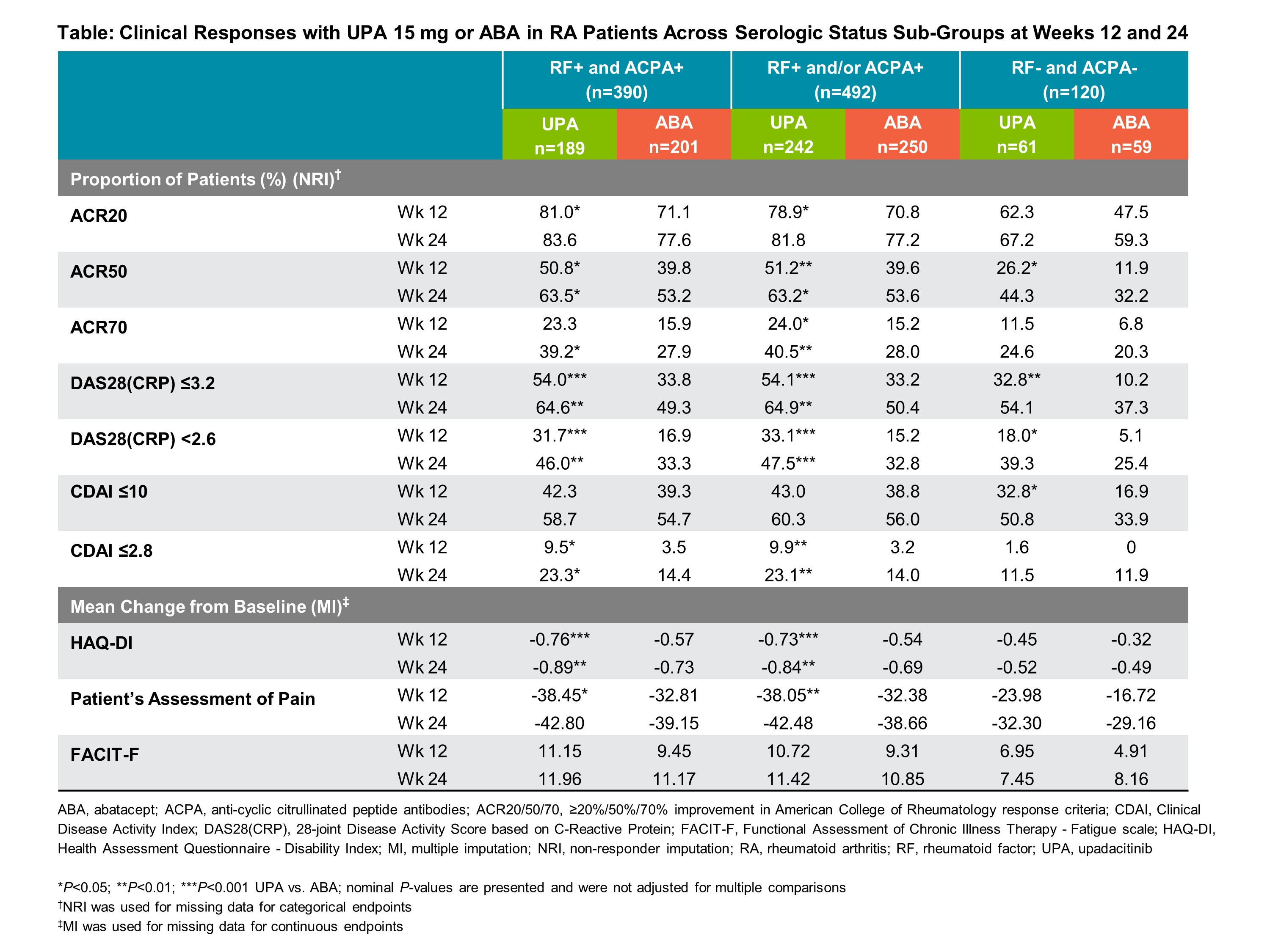
Methods: In SELECT-CHOICE (24-week, phase 3, double-blind, controlled trial), RA patients were randomized to oral UPA (15 mg once daily) or intravenous (IV) ABA (at day 1 and weeks 2, 4, 8, 12, 16, and 20; < 60 kg, 500 mg; 60-100 kg, 750 mg; >100 kg, 1000 mg).1 UPA patients also received IV placebo and ABA patients also received oral placebo. All patients continued stable background conventional synthetic DMARDs. Starting at week 12, background RA medications were adjusted or added if patients did not experience ≥20% improvement compared to baseline in tender and swollen joint counts at two consecutive visits. For this sub-group analysis, patients were categorized as follows: RF+ and ACPA+, RF+ and/or ACPA+, and RF- and ACPA-. Mean change from baseline in DAS28(CRP), Clinical Disease Activity Index (CDAI), ACR responses, HAQ-DI, patient’s assessment of pain, and Functional Assessment of Chronic Illness Therapy – Fatigue scale (FACIT-F) were evaluated at weeks 12 and 24. Statistical inference was conducted using Chi-square tests or analysis of covariance (ANCOVA) with non-responder imputation or multiple imputation used for missing data and nominal P-values shown.
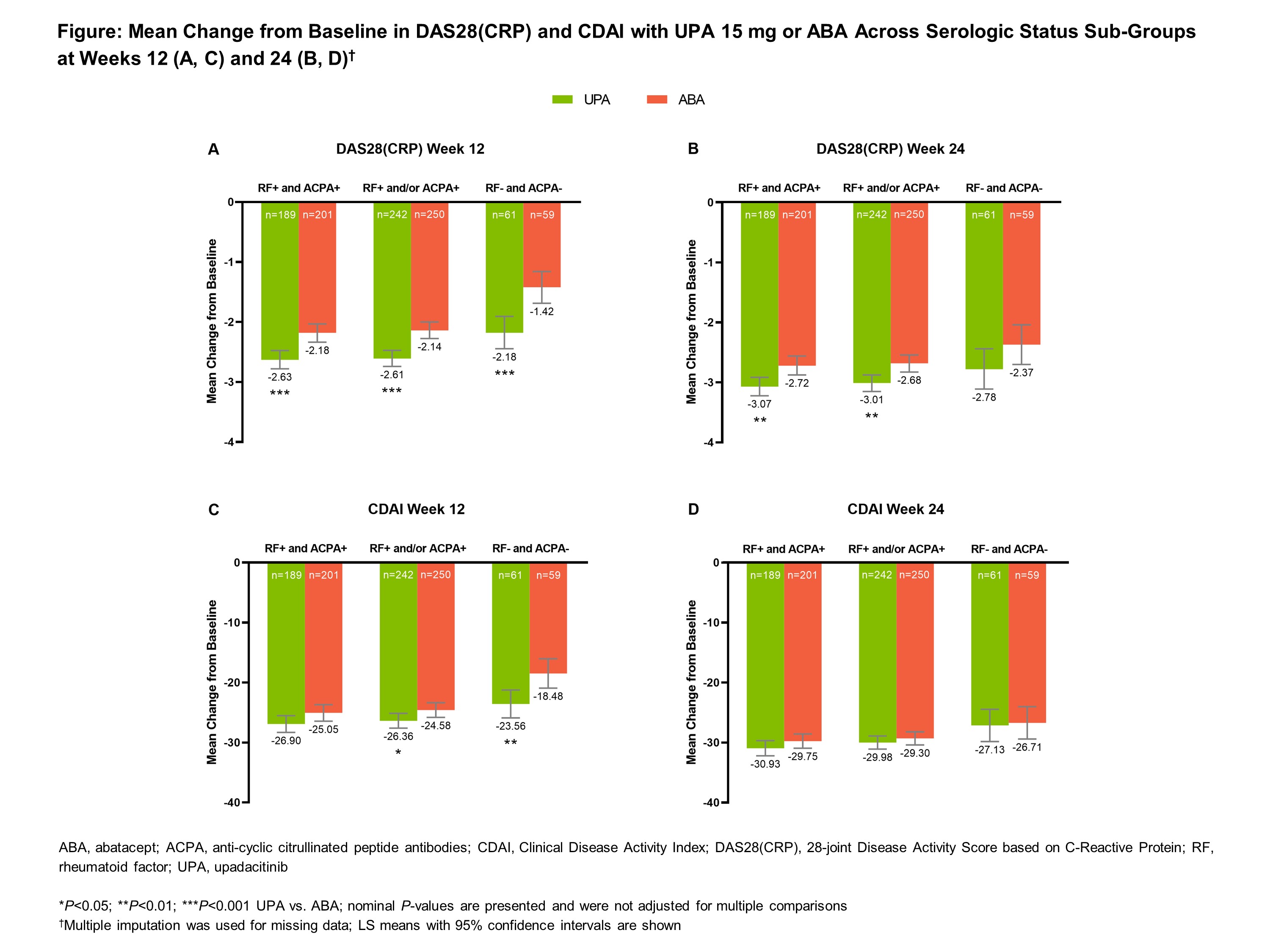
Results: Of the total population (N=612), the majority of patients were seropositive for RF and/or ACPA at baseline (80.4%) (Table). Most patients were female (~80%), ~55 years old, and one-third had previously experienced ≥2 biologic DMARDs. Mean change from baseline in DAS28(CRP) and CDAI were numerically higher with UPA vs ABA at weeks 12 and 24 across all sub-groups (Figure). Regardless of serologic status, UPA demonstrated numerically higher responses vs ABA for ACR 20/50/70 and proportions of patients in low disease activity and remission at both timepoints (Table). Mean change from baseline in the HAQ-DI and the patient’s assessment of pain was numerically higher with UPA compared to ABA across all sub-groups and timepoints. Clinical responses were generally numerically higher at week 24 compared to week 12, and for the seropositive groups compared to the seronegative group, with both UPA and ABA.
Conclusion: Across serologic statuses, clinical responses with UPA 15 mg vs ABA were numerically higher at weeks 12 and 24 among RA patients with prior inadequate response or intolerance to biologic DMARDs. In addition, clinical responses were numerically higher for seropositive patients compared to seronegative patients across all endpoints assessed, although the seronegative group had a smaller sample size in this post-hoc analysis.
References:
1. Rubbert-Roth A, et al. N Engl J Med. 2020;383(16):1511–21
2. Sokolove J, et al. Ann Rheum Dis. 2016;75:709-14
To cite this abstract in AMA style:
Rubbert-Roth A, Sparks J, Constantin A, Xavier R, Song Y, Suboticki J, Fleischmann R. Impact of Serologic Status on Clinical Responses to Upadacitinib or Abatacept in Patients with Rheumatoid Arthritis and Prior Inadequate Response to Biologic DMARDs: Sub-Group Analysis from the Phase 3 SELECT-CHOICE Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/impact-of-serologic-status-on-clinical-responses-to-upadacitinib-or-abatacept-in-patients-with-rheumatoid-arthritis-and-prior-inadequate-response-to-biologic-dmards-sub-group-analysis-from-the-phase/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-serologic-status-on-clinical-responses-to-upadacitinib-or-abatacept-in-patients-with-rheumatoid-arthritis-and-prior-inadequate-response-to-biologic-dmards-sub-group-analysis-from-the-phase/