Session Information
Date: Tuesday, November 10, 2015
Title: Systemic Sclerosis, Fibrosing Syndromes, and Raynaud's - Clinical Aspects and Therapeutics II
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: More than 90% of patients with Systemic Sclerosis (SSc) have gastroinstestinal (GI) involvement, commonly dysmotility causing complications such as gastroesophageal reflux and constipation. Treatment with prokinetic drugs is mostly modelled on their use in the general population, despite pathophysiological differences between GI disease in SSc and other etiology. Thus, our goal is to identify and appraise all studies evaluating the impact of prokinetics on GI outcomes in patients with SSc.
Methods: Three databases (Cochrane Central Register of Controlled Trials, Ovid MEDLINE, and Embase) were searched until November 27, 2013 using permutations of the terms “scleroderma” and “systemic sclerosis” combined with “prokinetic”, “anti-emetic”, and generic and trade names of individual drugs. Studies were included if they evaluated the impact of a prokinetic on any GI outcome in at least 5 adults (age > 18 years) with SSc, regardless of language or study type. Two reviewers independently evaluated all studies, and conflicts were resolved by a third.
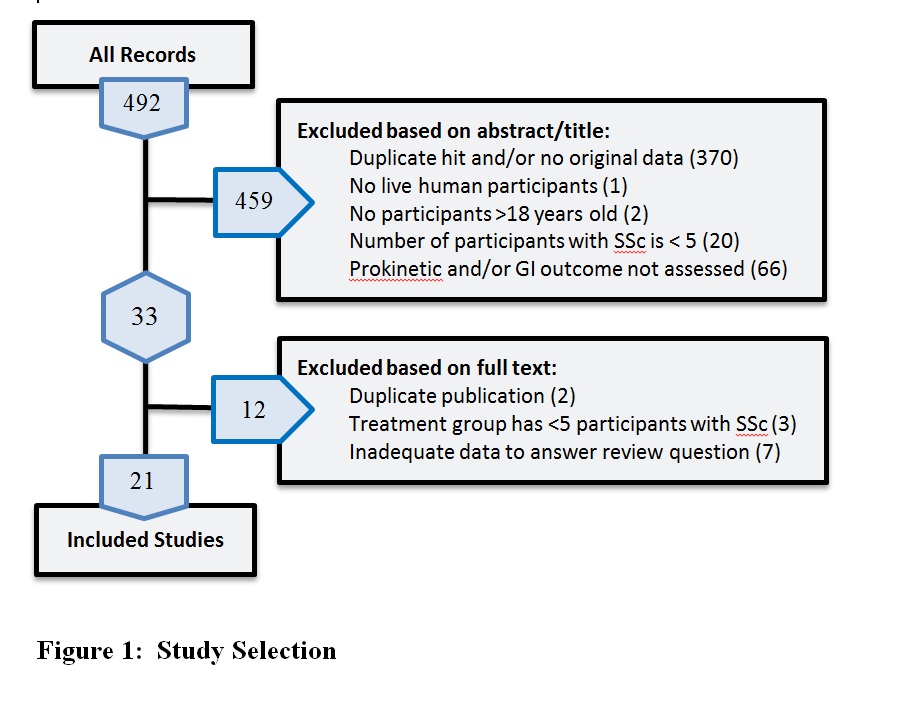
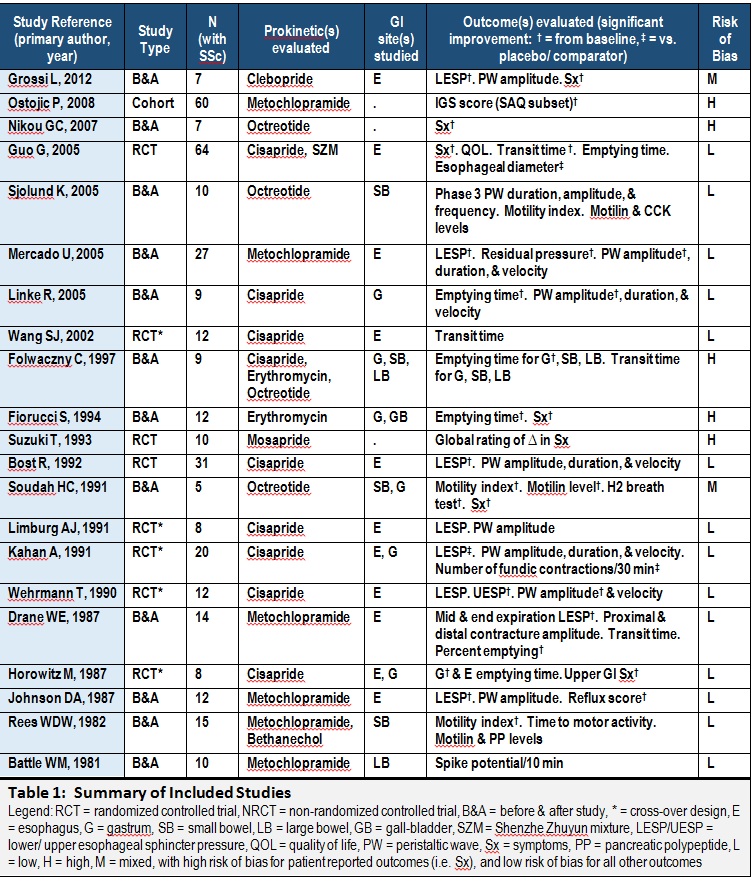
Results: Of 492 search results, 21 studies from 12 countries met our criteria (Figure 1). The sample sizes ranged from 5 to 64, with 362 participants in all (83% women), ranging in age from 30 to 80 years. Using Cochrane guidelines, 7 studies had high risk of bias in at least one domain for at least one outcome measure (Table 1). Six prokinetics were evaluated: cisapride in 9 studies, metoclopramide in 6, octreotide in 2, erythromycin in 2, and mosapride and clebopride in 1 study each. Only 2 studies evaluated >1 prokinetic. Outcomes included GI motility as evaluated by manometry (ex. esophageal sphincter pressure), scintigraphy (ex. gastric emptying time), and hydrogen breath tests; serum levels of motility-altering peptides (ex. motilin); and symptoms (ex. Index of Gastrointestinal Status). Each prokinetic was associated with a favourable outcome in at least half the studies that evaluated it, without serious adverse effects, except diarrhea with octreotide in one study. The heterogeneity in treatment administration, outcomes, and comparator groups precluded any meta-analyses.
Conclusion: Available studies suggest a favourable side-effect profile and impact of prokinetics on SSc-associated GI disease, but are limited by small numbers, lack of uniform outcome measures, and risk of bias. Evaluation of prokinetics in larger SSc cohorts using validated and standardized outcome measures are needed to assess the generalizability of these findings, and to determine the ideal type, dose, and duration of prokinetics for a given GI dysfunction in SSc.
To cite this abstract in AMA style:
Tisseverasinghe A, Kadhim A, Parmar A, Liu L, Johnson SR. Impact of Prokinetic Agents on Systemic Sclerosis-Associated Gastrointestinal Disease: A Systematic Review [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/impact-of-prokinetic-agents-on-systemic-sclerosis-associated-gastrointestinal-disease-a-systematic-review/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-prokinetic-agents-on-systemic-sclerosis-associated-gastrointestinal-disease-a-systematic-review/
