Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Mepolizumab (MEPO) proved its efficacy in the treatment of eosinophilic granulomatosis with polyangiitis (EGPA) in the randomized controlled MIRRA trial. The ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire is a novel, disease-specific tool, validated with the aim of assessing the impact of the disease and its treatment on the patients’ perspectives. The aim of our study is to prospectively evaluate the impact and the rapidity of the effect of MEPO on the AAV-PRO score and patient global assessment (PtGA) in a multicentre cohort of patients with EGPA.
Methods: Patients with EGPA satisfying the 2022 ACR/EULAR and/or MIRRA trial classification criteria and initiating treatment with MEPO were included. PtGA and AAV-PRO score were prospectively evaluated at MEPO initiation and after 7, 14, 30, 90 and 180 days of treatment. Predictors of improved AAV-PRO response during treatment with MEPO were also investigated.
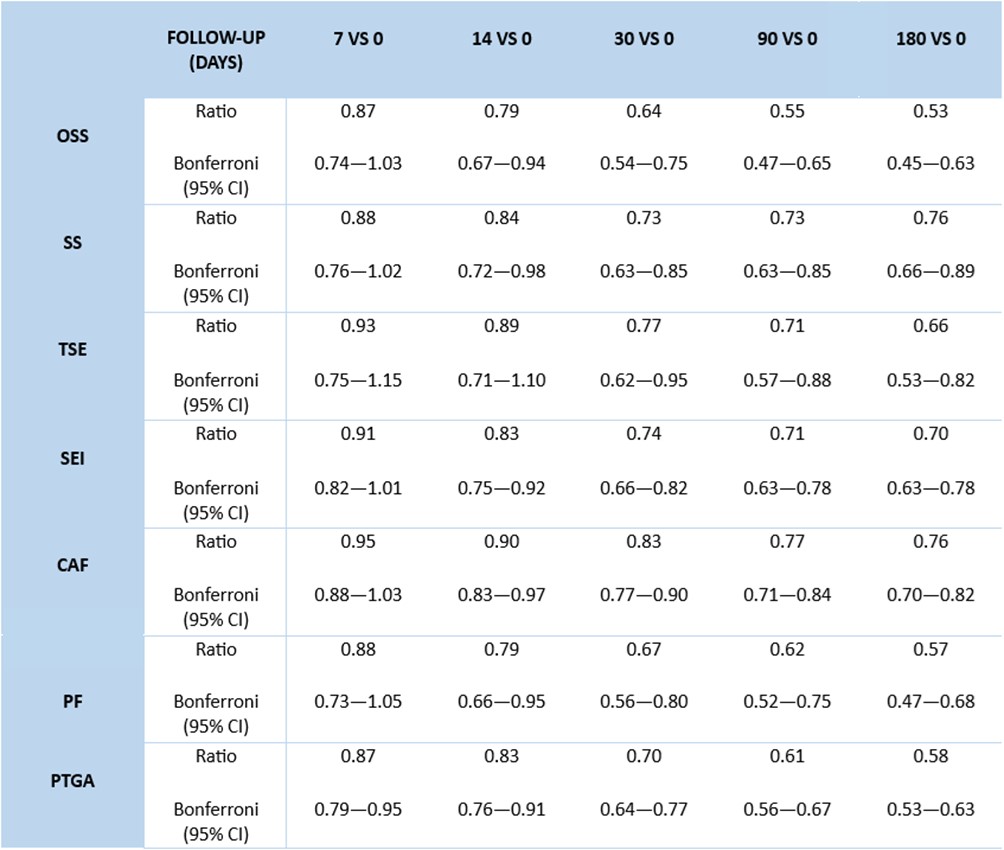
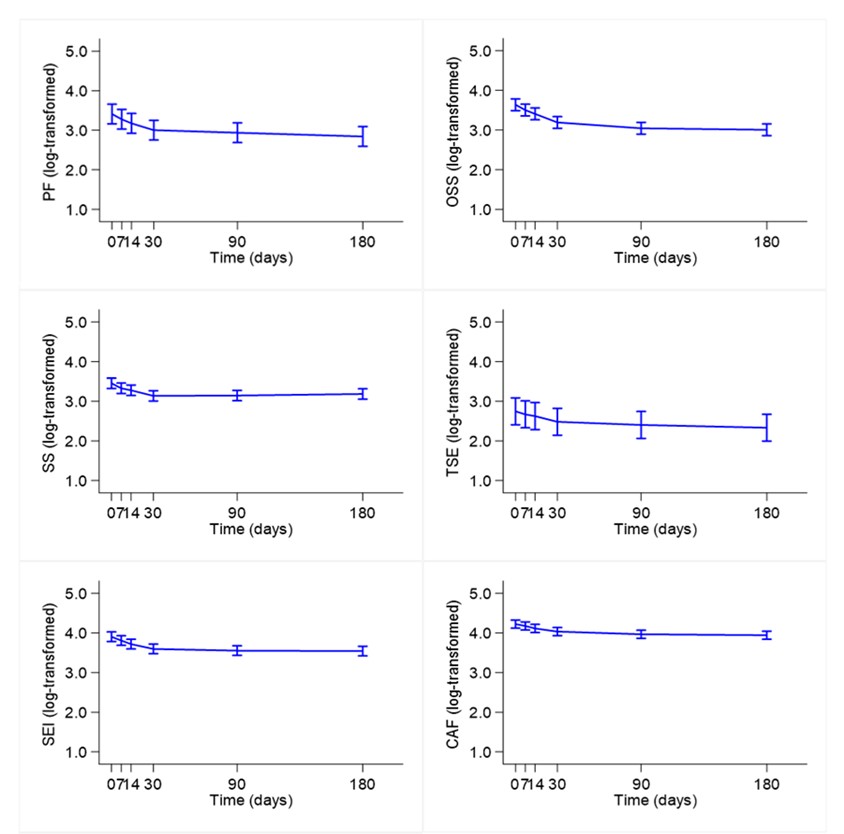
Results: Seventy patients were included in the study: female 54.3%, mean age at diagnosis 48.7±12.6 years, 20% ANCA-positive. Sixty-three patients (90%) had a history of relapsing disease. Forty-seven (67.1%) and 23 (32.9%) patients received MEPO 100 mg and 300 mg/4 weeks, respectively, with the main indication being refractory asthma and/or rhinosinusitis. Prednisone (PDN)-equivalent mean dose was 10.8±9.2 mg/day. At MEPO initiation, mean PtGA, AAV-PRO organ-specific symptoms, systemic symptoms, treatment side effects, social and emotional impact, concerns about the future and physical function domain 0-100 score were 59.1±21.2, 30.9±17.8, 25.5±18.6, 18.8±18.1, 32.6±21.9, 37.7±22.1 and 28.2±21.2, respectively. After 7 days, a statistically significant reduction of PtGA [ratio 0.87 (95% CI 0.87—0.95); Table 1] was observed. At 14 days, all AAV-PRO domains, except treatment side effects, showed a statistically significant improvement compared to baseline. Conversely, no relevant amelioration of the AAV-PRO domains was demonstrated between 30 and 180 days of follow-up (Figure 1). Previous history of vasculitic relapse, BVAS ≥3, C-reactive protein ≥5 mg/L and PDN-equivalent dose ≥10 mg/day at MEPO initiation were the main predictors of a better response of the AAV-PRO questionnaire during follow-up. MEPO 100 mg/4 weeks and female sex positively influenced organ-specific symptoms compared to MEPO 300 mg/4 weeks, although with a less significant improvement of the treatment side effects domain. Conversely, age, educational level and ANCA status at baseline had no impact on the AAV-PRO domains.
Conclusion: MEPO allows a quick and remarkable improvement of several aspects of health-related quality of life in patients with refractory EGPA.
To cite this abstract in AMA style:
Delvino P, Quartuccio L, Robson J, Alberici F, Bagnasco D, Berti A, Caminati M, Camoni M, Cid M, Conticini E, Costanzo G, de Moreuil C, Del Giacco S, Espigol-Frigole G, Ferretti V, Franceschini F, Iorio L, Kernder A, Klersy C, Lo Gullo A, Losappio L, Manna E, Maule M, Montecucco C, Simone N, Padoan R, Regola F, Ricciardi L, Schroeder J, Terrier B, Toniati P, Treppo E, Urban M, Vaglio A, Emmi G, Monti S. Impact of Mepolizumab on Patient-reported Outcomes in Eosinophilic Granulomatosis with Polyangiitis by Using the ANCA-associated Vasculitis Patient-reported Outcomes (AAV-PRO) Questionnaire: A European Multicentre Prospective Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-mepolizumab-on-patient-reported-outcomes-in-eosinophilic-granulomatosis-with-polyangiitis-by-using-the-anca-associated-vasculitis-patient-reported-outcomes-aav-pro-questionnaire-a-europea/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-mepolizumab-on-patient-reported-outcomes-in-eosinophilic-granulomatosis-with-polyangiitis-by-using-the-anca-associated-vasculitis-patient-reported-outcomes-aav-pro-questionnaire-a-europea/