Session Information
Date: Sunday, October 26, 2025
Title: (0337–0356) Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Janus kinase (JAK) inhibitors are an important treatment option for rheumatoid arthritis (RA) and psoriatic arthritis (PsA). By disrupting pro-inflammatory cytokine signaling (e.g., IL-6, TNF-α), they reduce inflammation and may potentially mitigate bone loss. Chronic inflammation in RA and PsA leads to systemic and local imbalances in bone remodeling, characterized by increased osteoclast activity and decreased osteoblast function. While JAK inhibitors have shown promise in counteracting these processes locally, clinical evidence regarding their effects on systemic bone health remains limited.We aimed to quantify the impact of JAK inhibitors on bone health in a real-world cohort of patients with RA and PsA.
Methods: Baseline data from patients with RA or PsA from the Rh-GIOP study, a prospective observational cohort at Charité–Universitätsmedizin Berlin, were included in this cross-sectional analysis. Bone health was assessed using DXA for bone mineral density (expressed as the minimum T-Score from lumbar spine and hip measurements), trabecular bone score (TBS; enCORE V18 iNsight®, Med-Imaps SA), and 3D structural parameters of the femur (3D-Shaper v2.12, 3D-Shaper Medical). Multivariable linear regression models were used to identify clinical, serological, and treatment-related factors associated with T-Score and TBS. JAK inhibitors were included in these models to evaluate their impact on outcomes, provided that a prior stable treatment period had been established. Missing data were addressed using multiple imputation with 10 replications.
Results: The study included 856 patients (mean age 64 years, 77% female, 92% postmenopausal), with 89 receiving JAK-inhibitors (33 baricitinib, 25 tofacitinib, 21 upadacitinib, 10 filgotinib). Osteoporosis was prevalent in 24% of patients, while the majority had either normal bone mineral density (BMD) or osteopenia. Treatment with JAK inhibitors was not significantly associated with T-Score (table 1). However, vertebral trabecular microarchitecture expressed by TBS, showed that JAK-inhibition was positively associated with TBS (β (95%CI)= +0.061 (0.021;0.101), p=0.003), suggesting a potential positive impact on trabecular microarchitecture.
Conclusion: In this real-world cohort, JAK inhibitors were associated with positive impact on trabecular microarchitecture. These findings suggest that a broad cytokine inhibition may support bone health. Longitudinal studies are required to confirm these observations and evaluate their implications for fracture prevention and individualized treatment strategies.
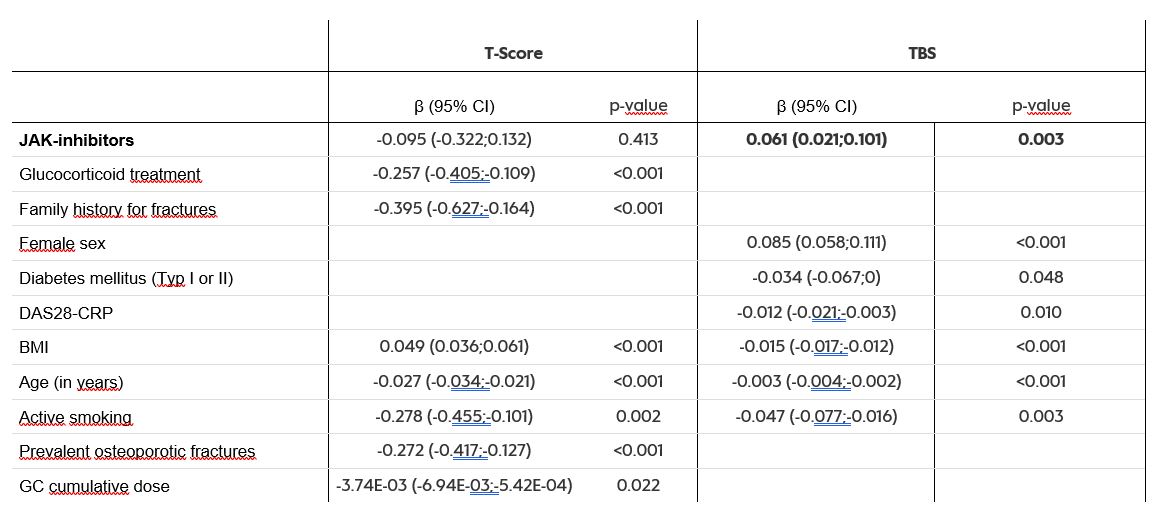 Table 1: Results from the multivariable linear regression model with backward selection for minimum T-Score and trabecular bone score (TBS) forcing JAK inhibitors into the model
Table 1: Results from the multivariable linear regression model with backward selection for minimum T-Score and trabecular bone score (TBS) forcing JAK inhibitors into the model
JAK : Janus kinase, BMI : Body Mass Index, CI : confidence interval, CRP : C-reactive Protein, DAS28 : Disease-activity score 28, GC : Glucocorticoids
To cite this abstract in AMA style:
Wiebe E, Huscher D, Boyadzhieva Z, Palmowski A, Hermann S, Muche B, Kleyer A, Simon D, Krönke G, Hoff P, BUTTGEREIT F. Impact of Janus Kinase Inhibitors on Bone Mineral Density and Microarchitecture in Rheumatoid and Psoriatic Arthritis: Insights from a Real-World Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-janus-kinase-inhibitors-on-bone-mineral-density-and-microarchitecture-in-rheumatoid-and-psoriatic-arthritis-insights-from-a-real-world-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-janus-kinase-inhibitors-on-bone-mineral-density-and-microarchitecture-in-rheumatoid-and-psoriatic-arthritis-insights-from-a-real-world-cohort/
