Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS) is strongly associated with the genetic marker HLA-B27. In patients with AS, negative HLA-B27 status is a predictor of worse response to tumor necrosis factor inhibitors.1 Approximately 80%-90% of Caucasian patients with AS express HLA-B27 compared with < 8% of the Caucasian population; these trends are similar in Chinese patients. The objective of this study is to analyze the impact of HLA-B27 status on clinical outcomes at Week 16 in a racially diverse population of patients with AS treated with secukinumab vs placebo.
Methods: Patients with AS were pooled from the MEASURE 1-5 studies (NCT01358175, NCT01649375, NCT02008916, NCT02159053, and NCT02896127) and stratified by HLA-B27 status. All trials included patients who received secukinumab 150 mg every 4 weeks with or without an initial loading dose (10 mg/kg IV at Weeks 0, 2, and 4 or 150 mg SC at Weeks 0, 1, 2, and 3) or placebo control. MEASURE 3 included patients receiving secukinumab 300 mg every 4 weeks following the initial IV loading dose. A large proportion of patients enrolled in MEASURE 5 were from China. Efficacy at Week 16 was determined by the proportion of patients reporting 20% improvement in Assessment of SpondyloArthritis international Society (ASAS20), ASAS40, ASAS5/6, ASAS partial remission, 50% improvement in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), and Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) of < 2.1 or < 1.3. All outcomes were compared within HLA-B27 strata for secukinumab vs placebo using logistic regression at Week 16 with nonresponder imputation for missing data. All analyses were for hypothesis generation, without adjustment for multiple comparisons.
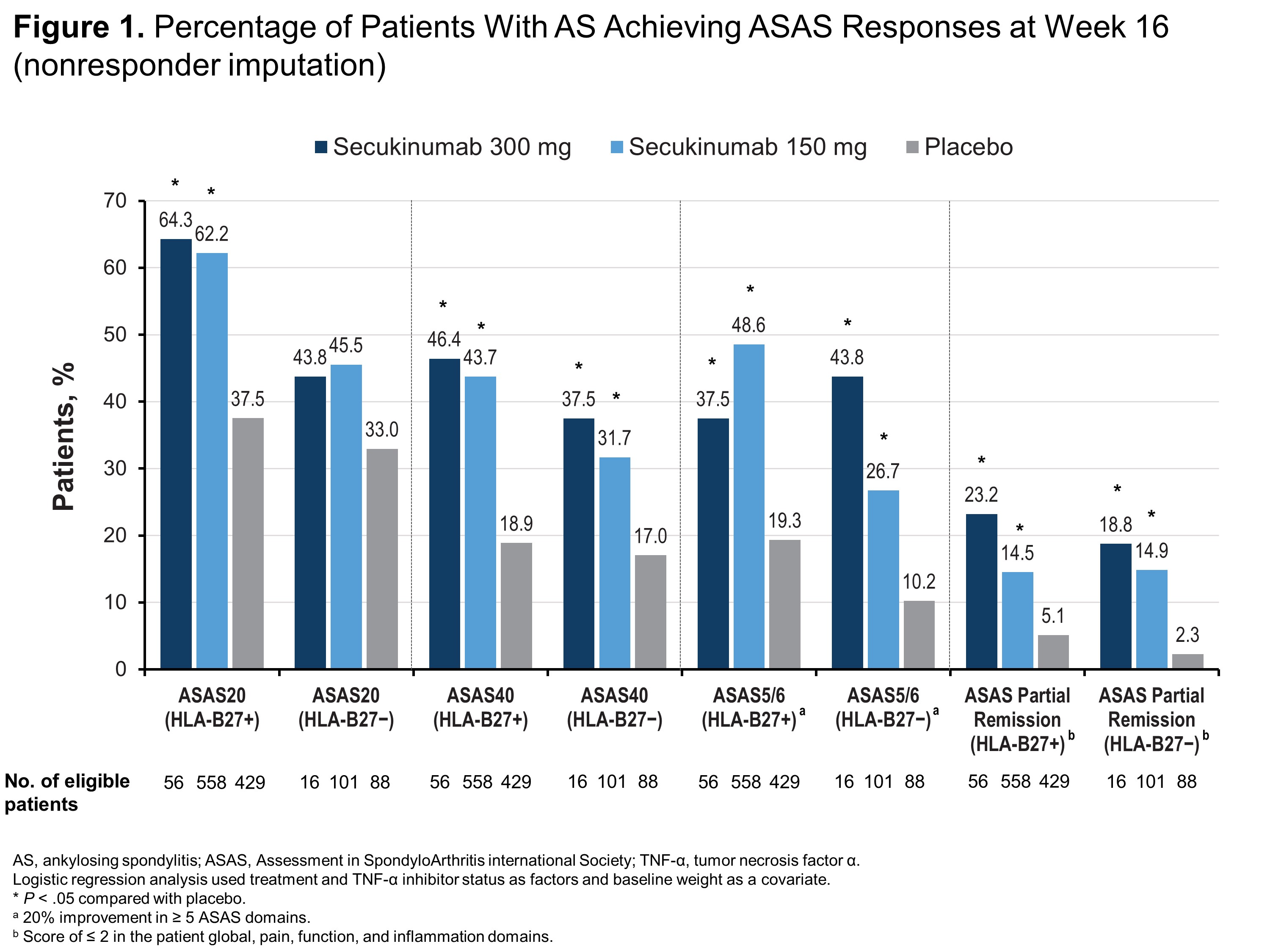
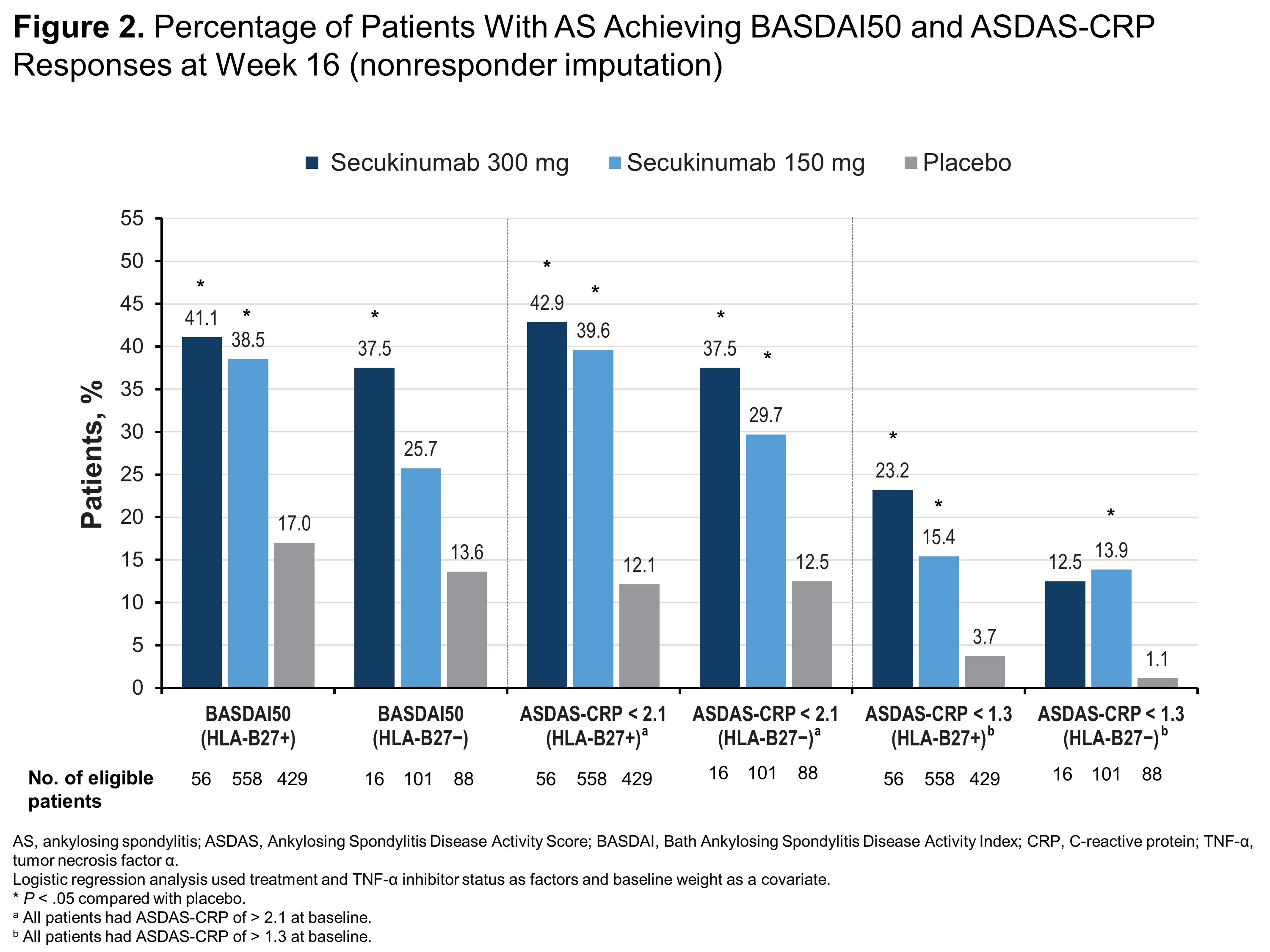
Results: Of 1248 patients included in this pooled analysis, 1043 (83.6%) were HLA-B27+ and 205 (16.4%) were HLA-B27−. Compared with HLA-B27− patients, HLA-B27+ patients were younger (38.6 vs 45.0 years) and more frequently male (77.1% vs 48.8%). Regardless of HLA-B27 status, patients receiving any dose of secukinumab were significantly more likely to report ASAS40, ASAS5/6, ASAS partial remission (Figure 1) and ASDAS-CRP of < 2.1 (Figure 2) than those receiving placebo (P < .05 for all comparisons). HLA-B27+ patients receiving any dose of secukinumab were also significantly more likely to report ASAS20, BASDAI50, and ASDAS-CRP responses vs those receiving placebo (P < .05) (Figures 1 and 2), although HLA-B27− patients experienced at least numerical improvements vs placebo in these measures. Among HLA-B27− patients, only those receiving secukinumab 300 mg were significantly more likely to report BASDAI50 response vs those receiving placebo, and only patients receiving secukinumab 150 mg were significantly more likely to have ASDAS-CRP of < 1.3 vs placebo (P < .05 for all comparisons) (Figure 2).
Conclusion: In a large pooled population of patients with AS including patients from China, secukinumab was effective regardless of HLA-B27 status, although HLA-B27+ patients experienced increased therapeutic benefit compared with HLA-B27− patients.
Reference:
- Alazmi M, et al. Arthritis Care Res (Hoboken). 2018;70:1393-1399.
To cite this abstract in AMA style:
Deodhar A, Mease P, Poddubnyy D, Strand V, Machado P, Shete A, Meng X, Magrey M. Impact of HLA-B27 Status on Clinical Outcomes in Patients with Ankylosing Spondylitis Treated with Secukinumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-hla-b27-status-on-clinical-outcomes-in-patients-with-ankylosing-spondylitis-treated-with-secukinumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-hla-b27-status-on-clinical-outcomes-in-patients-with-ankylosing-spondylitis-treated-with-secukinumab/