Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: End-stage renal disease (ESRD) is a serious complication of lupus nephritis (LN), yet the effect of guideline-concordant care on this outcome is unclear. Despite the availability of treatment guidelines, adherence remains inconsistent. This study examines the association between guideline-concordant care and adverse renal outcomes in pediatric Medicaid patients with childhood-onset LN.
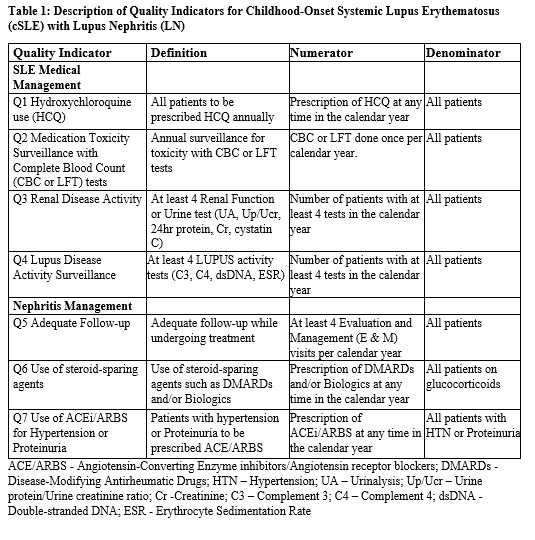
Methods: Using Medicaid via Marketscan data from 2011-2022, we identified individuals with LN between the ages of 5 and 21. Patients are required ≥ 3 or more SLE ICD-9 or ICD-10 codes in outpatient visit claims or hospital discharge diagnosis codes at least 30 days apart and have ≥3 ICD-9/ICD-10 for nephritis and a procedure code for renal biopsy. The exposure was the 7 quality indicators (Table 1), and the outcome was the adverse renal outcome (end-stage renal disease, dialysis, or kidney transplant). Descriptive statistics were used to characterized baseline data (Table 2). We compared quality indicator adherence between patients with and without adverse renal outcomes (Table 3). Hazard Ratio was obtained for adverse renal outcomes with quality indicators exposures that reached statistical significance (p< 0.05). Annual adjusted rates of adverse renal outcomes were calculated using Poisson regression with robust standard errors. For patients who had multiple years of exposure, we modeled the impact of cumulative quality indicator exposures on renal outcomes with Poisson regression.
Results: We identified 420 children with prevalent childhood-onset LN. The only statically significant difference noted was race and ethnicity where 12.6% were Hispanic, 53.6% Black, 14.3% White, 11.6% Unknown, 8.6% Other (p=0.04) . Adherence to quality indicators was moderate to high (HCQ use: 77.6%, medication toxicity: 88.6 %, renal disease activity monitoring: 87.7%, lupus disease activity monitoring: 63.5%, adequate follow-up: 81.2%, use of steroid-sparing agent: 85.6%, use of ACEi or ARB 68.5%. (Table 3). Use of ACEi/ARBs for HTN or Proteinuria was lower for those with adverse renal outcomes (69.39% vs 51.85%, p=0.007). The risk of adverse renal outcomes is 45% lower for patients with LN with HTN or proteinuria on an ACEi/ARB than those without (age, sex, race adjusted HR 0.55 [95% CI 0.32-0.96). When looking at the cumulative effect of having the ACEi/ARB quality indicator present 2 and 3+ years was statistically significant. Annual Rates of adverse renal outcome at 0 years was statistical higher compared to year 2 and 3+ year respectively (age, sex, race adjusted rates 11.0 [95% CI 6.5-18.6 ) vs 4.7 [ 95% CI 2.3-9.6 ] and 5.6 [95% CI 3.1-10.0]).
Conclusion: Use of ACE inhibitors or ARBs was associated with a lower risk of adverse renal outcomes in children with lupus nephritis, with greater cumulative exposure linked to further risk reduction. Additionally, hydroxychloroquine use showed a trend toward improved renal outcomes, suggesting potential benefit. These findings support the importance of consistent, guideline-based medication use in improving long-term renal health in this population.
.jpg) Data is presented as percentage of number X person years with QI among number of person X exposure years
Data is presented as percentage of number X person years with QI among number of person X exposure years
To cite this abstract in AMA style:
Akinsete A, Soroka O, Rajan M, Onel K, Safford M, Navarro-Millan I. Impact of Guideline Concordant Care of Renal Outcomes in Childhood-Onset Lupus Nephritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-guideline-concordant-care-of-renal-outcomes-in-childhood-onset-lupus-nephritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-guideline-concordant-care-of-renal-outcomes-in-childhood-onset-lupus-nephritis/

.jpg)