Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: It is recommended that vaccinations should be performed prior to start methotrexate (MTX) knowing that delaying initiation of background therapy may have an impact on the progression of RA. What is the real impact of delaying initiation of MTX by 1 month on the outcome of RA at 1 year ?
Methods: The VACIMRA study is a prospective, randomized, parallel-group, multicenter trial comparing the vaccine protection obtained in patients with rheumatoid arthritis according to the 1-month delay between anti-pneumococcal vaccine PCV13 and methotrexate initiation in one arm, versus immediate introduction of MTX following vaccination in the other arm. We analyzed disease activity based on DAS28-ESR at baseline (M0), 1, 2, 3, 6 and 12 months between the 2 groups. For structural progression, we performed a radiographic analysis of 79 RA patients included in the Montpellier center at baseline, 6 and 12 months. This analysis was performed by the same physician two times, blinded to the patient’s group. Structural damage progression at 6 months and 1 year was assessed according to van-der-Heijde-modified Sharp score (vSHS) on radiographs performed at inclusion, at 6 and 12 months of follow-up. Comparisons of the means of activity scores and radiographic scores were made with the non-parametric Wilcoxon-Mann-Whitney test.
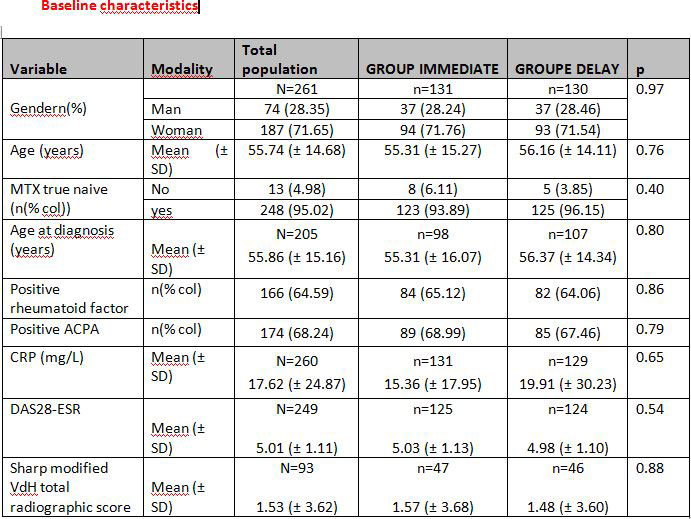
Results: Of the 276 patients randomized, 261 could be analyzed (131 in the IMMEDIATE group and 130 in the DELAY group). At inclusion, there were no significant differences in demographic, disease activity (DAS28-ESR), biological and radiographic characteristics between the 2 groups.
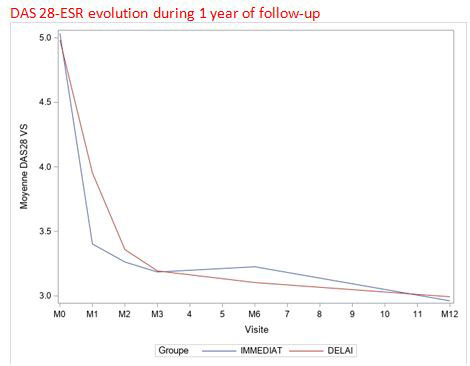
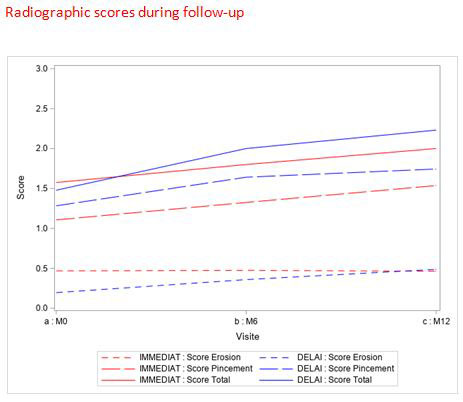
There was a significant difference in the means of DAS28-ESR at 1 month between the DELAY and IMMEDIAT groups (3.96 ± 1.46 vs 3.41 ± 1.33; p< 0.001, respectively). There was no significant difference in the means of DAS28-ESR between the 2 groups at 3 months (3.19± 1.46 in the 2 groups p< 0.91), at 6 months (3.11 ± 1.42 vs 3.24 ± 1.43; p=0.46, respectively) and at 12 months (2.96 ± 1.34 vs 2.98 ± 1.26p=0.89) (Graphic). Similarly, there was no significant difference in mean radiographic scores at 6 months (2.00 ± 4.41 vs. 1.80 ± 4.03 p=0.81) or at 12 months (2.23 ± 4.86 vs. 2.00 ± 4.07 p=0.93).
Regarding structural progression, radiographic scores had good intraobserver reproducibility (ICC=0.98). There was no significant variation between radiographic scores at 6 months compared to baseline in either group (mean difference 0.21 ± 0.52 vs. 0.36 ± 1.01, p=0.90) nor at 12 months compared to baseline (mean difference 0.40 ± 1.06 vs. 0.62 ± 1.58, p=0.85).
Conclusion: In patients with rheumatoid arthritis, initiation of methotrexate 1 month after PCV13 vaccination has no significant impact on RA activity and structural outcome at 1 year. Performing vaccinations 1 month before starting MTX can be proposed without significant impact on RA outcome at 1 year.
To cite this abstract in AMA style:
Than T, Dernis E, BROCQ O, Fautrel B, HUGUET H, Euller-Ziegler L, BUSTAMENTE-CENCI L, Vittecoq O, PICOT M, Lukas C, DAIEN C, Hua C, Genty M, Che H, REMY-MOULARD A, LIOTE F, Soubrier M, GAUJOUX-VIALA C, Constantin A, Saraux A, Ducourau E, RIST S, SALLIOT C, IBRAHIM-NASSER N, qUINTEN C, Goeb V, Morel J. Impact of Delaying Initiation of Methotrexate by One Month on the Outcome of RA at One Year [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-delaying-initiation-of-methotrexate-by-one-month-on-the-outcome-of-ra-at-one-year/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-delaying-initiation-of-methotrexate-by-one-month-on-the-outcome-of-ra-at-one-year/