Session Information
Date: Sunday, November 13, 2022
Title: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
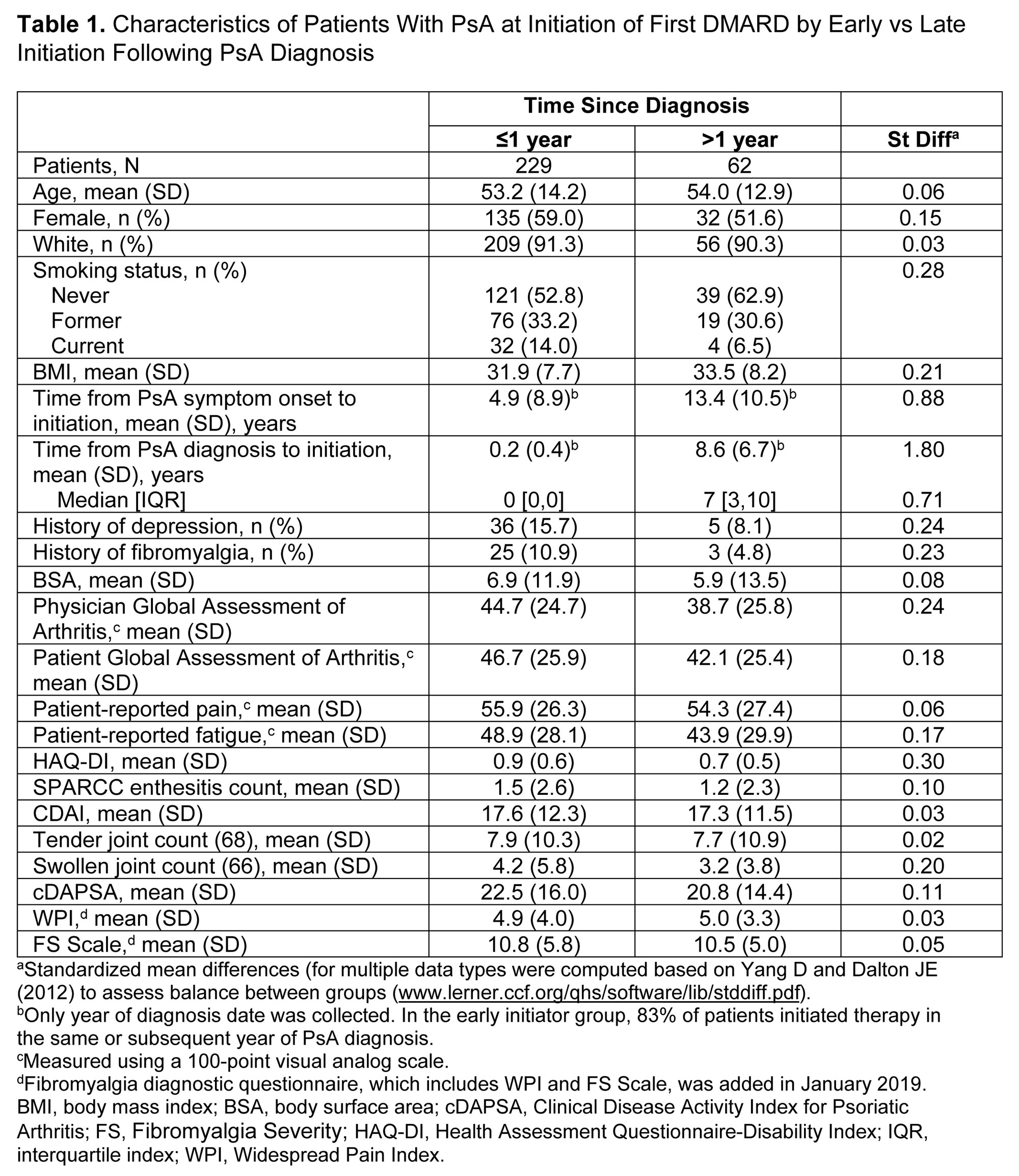
Background/Purpose: There is evidence that a delay between psoriatic arthritis (PsA) symptom onset and diagnosis contributes to worse outcomes, yet the impact of delayed DMARD therapy after diagnosis is unknown. This study describes patient characteristics and compares clinical and patient-reported outcomes (PROs) between patients with PsA in the CorEvitas PsA/Spondyloarthritis (SpA) Registry who started DMARD therapy earlier vs later in their disease course.
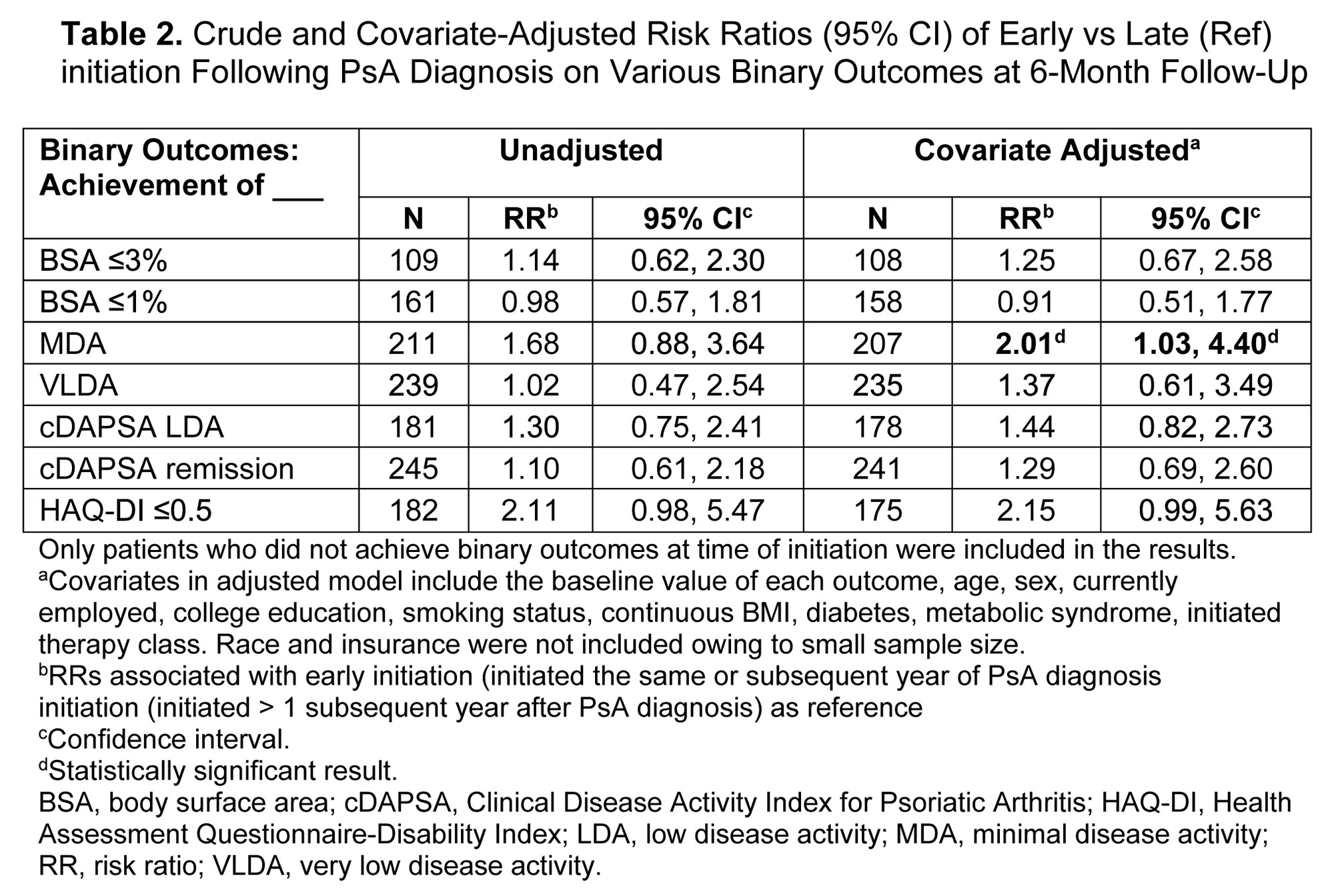
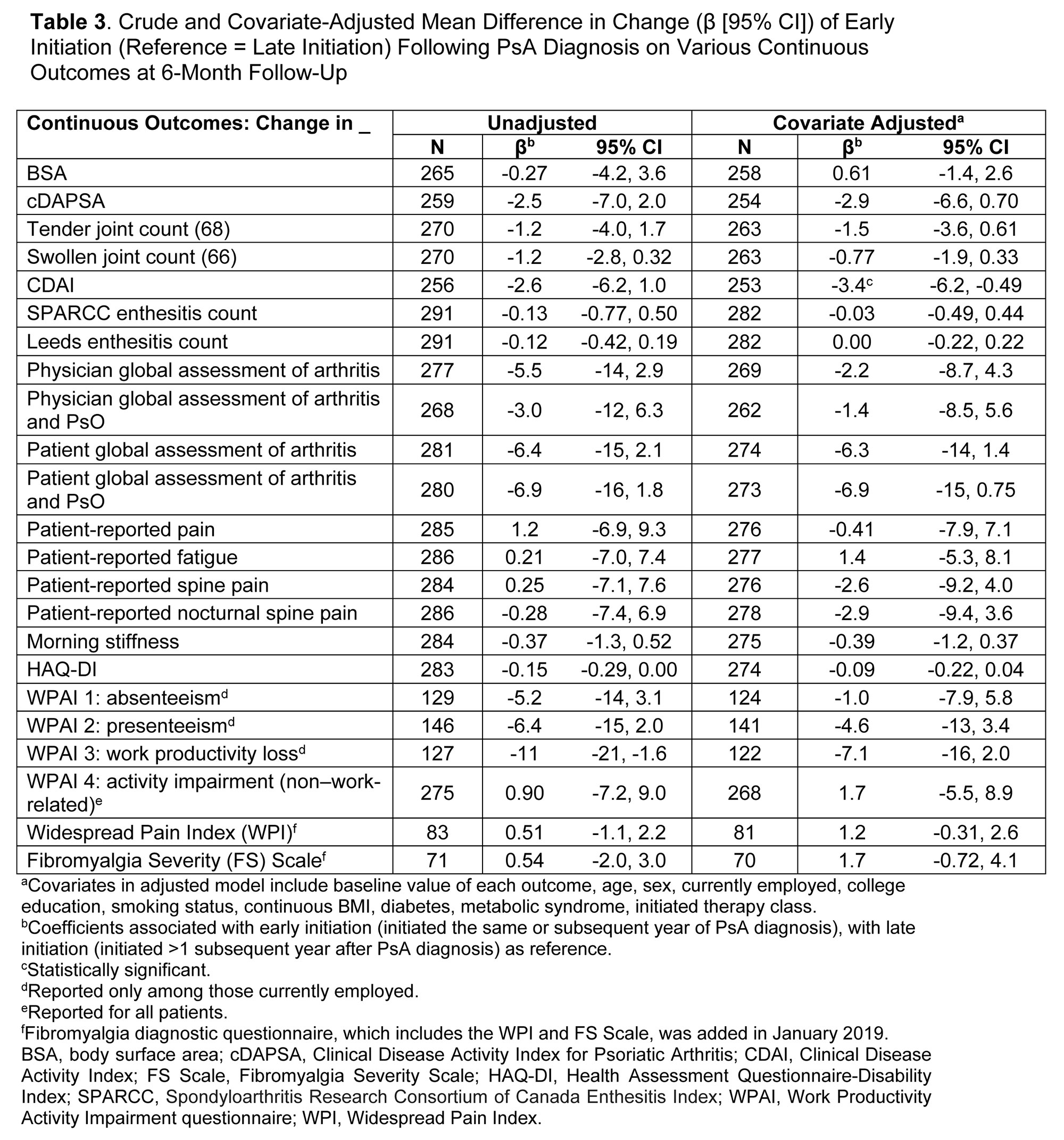
Methods: Patients enrolled in the registry between April 2013 and February 2022 who initiated their first DMARD at a CorEvitas visit (index) were included. At index visit, year of PsA diagnosis and of first DMARD initiation were ascertained. Patients were classified as early initiators (initiated the same or subsequent year of PsA diagnosis) or as late initiators (initiated >1 subsequent year after PsA diagnosis). Disease activity measures and PROs were collected at index visit and at 6 (± 3) months of follow-up. The associations of early vs late DMARD initiation with disease activity and PROs at 6 months were calculated using unadjusted and adjusted linear and Poisson regression. Multivariate models were adjusted for age, sex, employment, education, smoking, body mass index (BMI), diabetes, metabolic syndrome, initiated therapy class, and baseline value of outcome. Mean change in continuous outcomes (β, 95% confidence interval [CI]) and risk ratios (RR) and 95% CIs for binary response outcomes are reported. Sensitivity analyses were conducted defining delay of therapy as time from symptom onset.
Results: Of 291 patients included, 229 (79%) were early and 62 (21%) were late initiators. Among early initiators, 83% initiated therapy in the same or subsequent year of PsA diagnosis, and among late initiators, 50% initiated therapy 2–7 years after PsA diagnosis. Mean age at DMARD initiation was 53.2 and 54.0 years for early and late initiators, respectively. Early and late initiators differed according to smoking status (early vs late current smokers, 14.0% vs 6.5%), history of depression (15.7% vs 8.1%), and history of fibromyalgia (10.9% vs 4.8%). Early initiators reported greater severity of disease activity and worse PROs at index visit than late initiators (Table 1). From index to 6-month follow-up visit, improvements were seen across all disease activity measures and PROs in both groups, with larger improvements observed in the early initiators in the unadjusted models (Tables 2 and 3). In adjusted analyses, early initiation tended to be associated with better response, although only achievement of MDA (RR [95% CI] = 2.01 [1.03, 4.40]; Table 2) and mean change in CDAI (β [95% CI] = −3.4 [-6.2, −0.49]; Table 3) were statistically significantly different. In sensitivity analyses based on time from symptom onset, results were similar.
Conclusion: In this study of patients with PsA initiating their first DMARD therapy, both early and late initiators experienced improvements across all disease activity measures and PROs up to 6 months post-initiation. However, findings indicate that a better response, including MDA, may be achieved in patients initiating DMARD therapy within 1 year of diagnosis vs delaying treatment initiation.
To cite this abstract in AMA style:
Mease P, Nowak M, Choi J, Lehman T, Sreih A, Blachley T, Dube B, Ho K, Middaugh N, Ogdie A. Impact of Delay of Treatment with Disease Modifying Antirheumatic Drugs (DMARDs) in Psoriatic Arthritis: Lessons from the CorEvitas PsA/SpA Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-delay-of-treatment-with-disease-modifying-antirheumatic-drugs-dmards-in-psoriatic-arthritis-lessons-from-the-corevitas-psa-spa-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-delay-of-treatment-with-disease-modifying-antirheumatic-drugs-dmards-in-psoriatic-arthritis-lessons-from-the-corevitas-psa-spa-registry/