Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SEL-212 is a once-monthly, investigational, two-component infusion therapy consisting of pegadricase (SEL-037, a pegylated uricase) and immune-tolerizing nanoparticles containing sirolimus (SEL-110), for the treatment of patients with chronic gout refractory to conventional therapy. Formation of anti-drug antibodies (ADAs) impairs serum uric (sUA) acid reduction and may predispose to infusion reactions (IRs). ADA formation is a significant limiting factor for the only approved uricase for treatment of gout (Lipsky PE, et al. Arthritis Res Ther 2014;16:R60). Hence, mitigation of ADAs is key in maintaining patients on treatment. Here, ADAs formed in response to SEL-212 treatment and their associations with treatment outcomes are described.
Methods: The DISSOLVE I (NCT04513366) and DISSOLVE II (NCT04596540) Phase 3 trials investigated efficacy and safety of SEL-212. In both studies, participants were randomized 1:1:1 between two doses of SEL-212 (high-dose [HD]: sequential infusions of 0.15 mg/kg SEL-110 and 0.2 mg/kg SEL-037; low-dose SEL-212 [LD]: sequential infusions of 0.10 mg/kg SEL-110 and 0.2 mg/kg SEL-037) and placebo. SEL-212 or placebo were administered every 28 days for up to 6 treatment periods (TPs) in DISSOLVE II, or up to 12 TPs in DISSOLVE I. Titers of ADAs against polyethylene glycol (PEG), uricase, and pegadricase, i.e. ADAs recognizing specifically the PEGylated uricase, were measured using immunoassays at baseline (BL) and during each TP. The relationships of ADAs with sUA were analyzed for the pooled DISSOLVE population from BL to TP6, and possible associations with ADAs were investigated for all IRs.
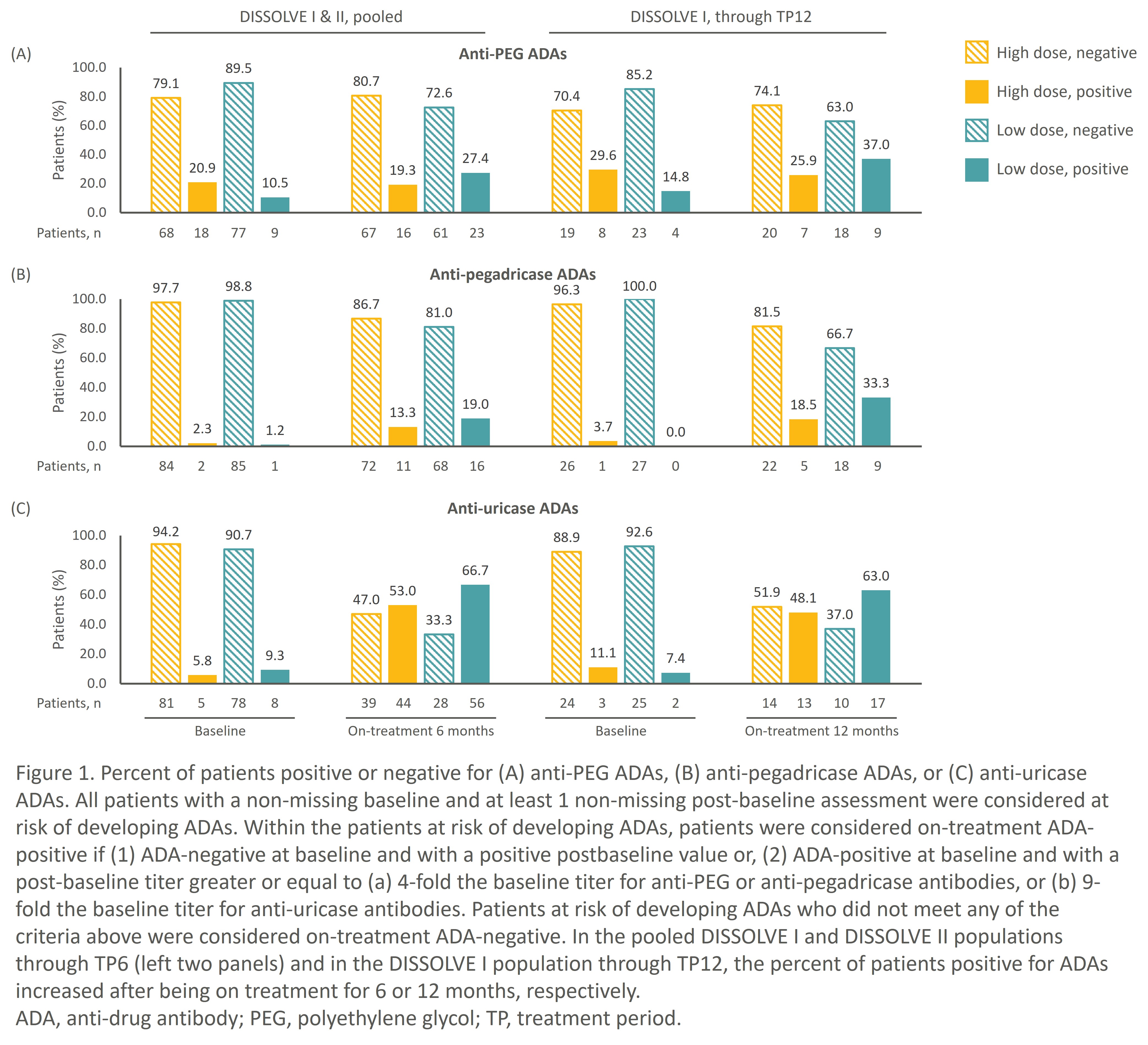
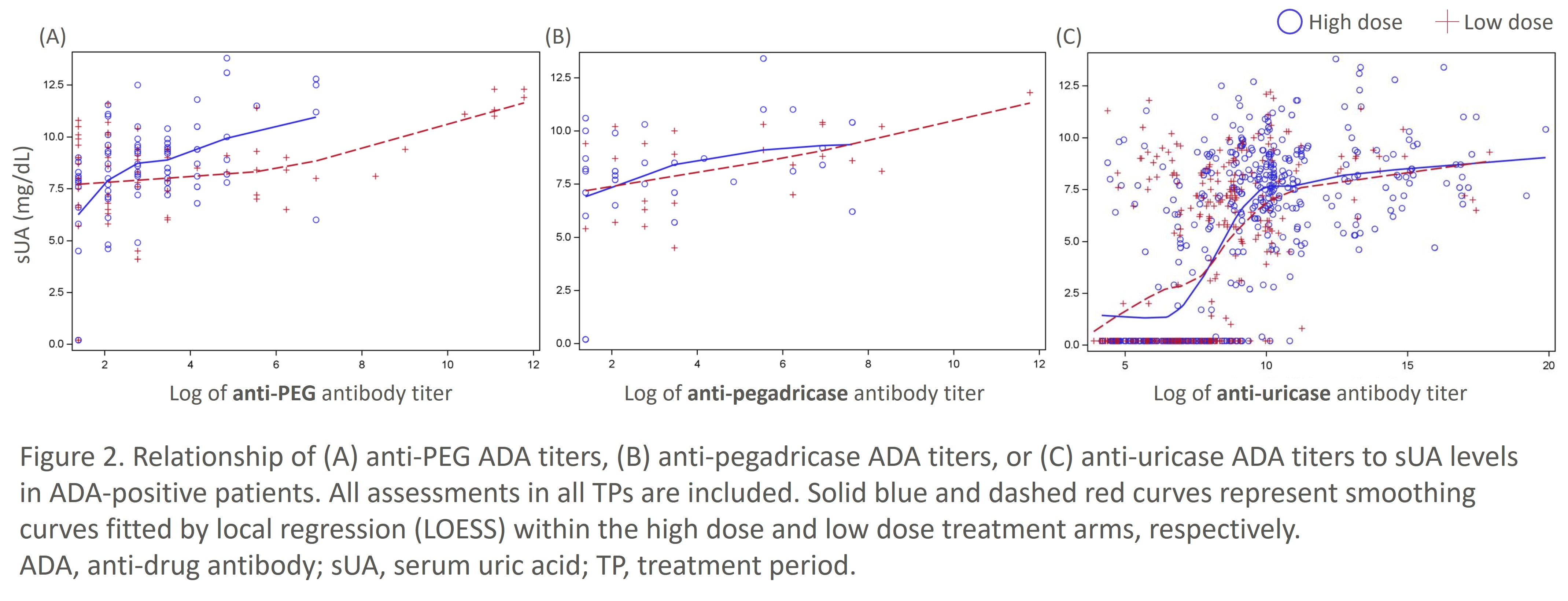
Results: In the pooled DISSOLVE population and DISSOLVE I cohort treated through TP12, fewer patients in the HD group developed ADAs compared to the LD group (Fig. 1). Moreover, the proportion of patients positive for anti-PEG ADAs did not increase markedly through TP12 in the HD group, demonstrating mitigation of ADAs by SEL-110. Anti-uricase ADAs were the most common ADAs observed primarily during the first 6 TPs, although only high-titer anti-uricase ADAs correlated with loss of sUA control (Fig. 2). Anti-PEG and anti-pegadricase ADAs were infrequently detected.
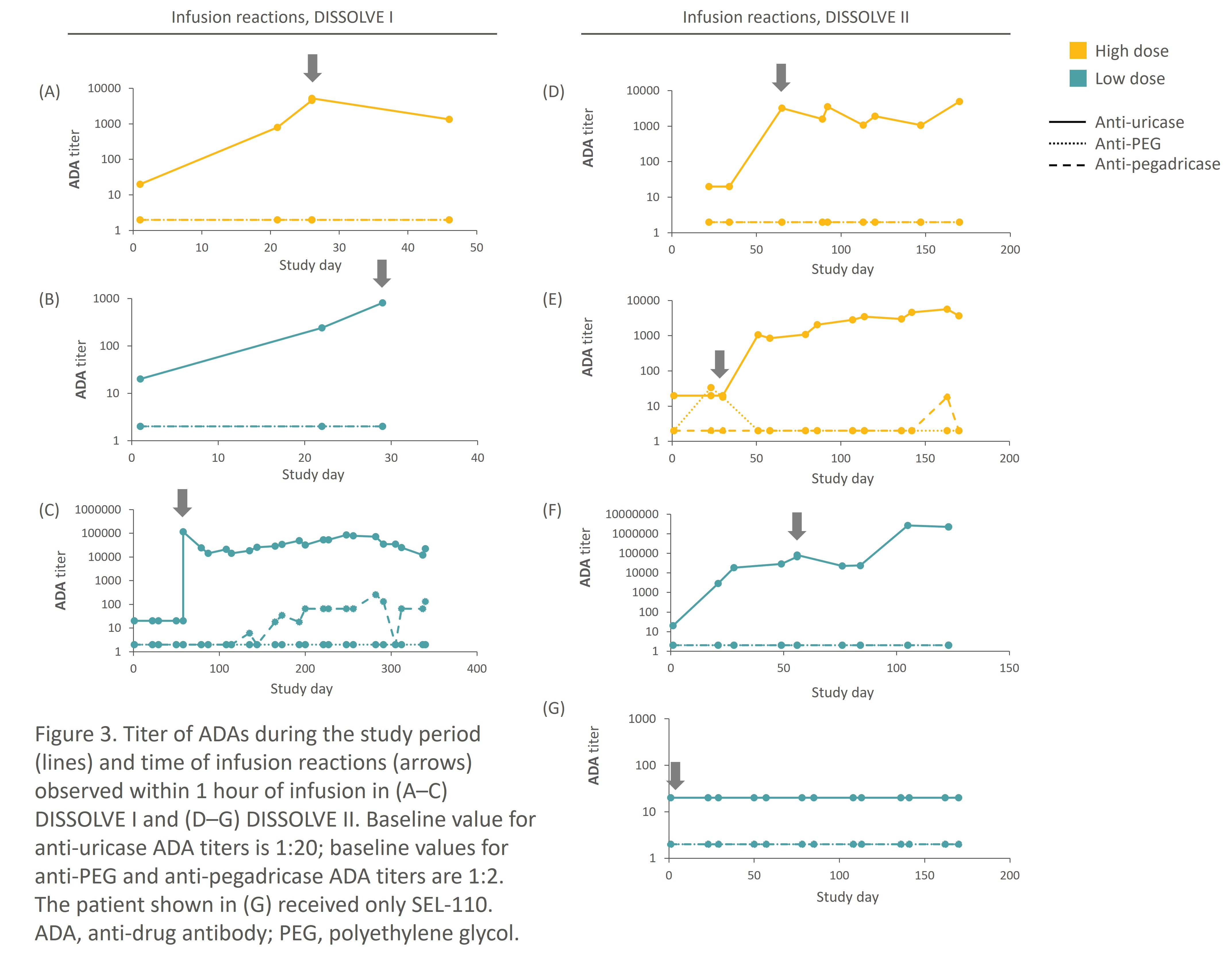
In the pooled DISSOLVE population, while 53% and 67% of patients on HD and LD SEL-212 were on-treatment positive for anti-uricase ADAs at TP6, respectively (Fig. 1), only 3 patients in each group had IRs within an hour of SEL-037 administration. All IRs occurred within the first 3 infusions and coincided with anti-uricase titers. No increases in anti-PEG or anti-pegadricase ADA titers were detected at the time of the IRs. Only one patient had an infusion reaction to the SEL-110 component (Fig. 3) – this occurred with the patient’s first exposure.
Conclusion: In the DISSOLVE studies, SEL-110 mitigated ADA formation for up to 12 months of treatment, allowing for sUA control in patients with chronic gout refractory to conventional therapy treated with SEL-212. Anti-uricase ADAs were the most common, as expected. However, loss of sUA control was typically restricted to high-titer anti-uricase ADAs and IRs were few, making this combination therapy a promising option for patients with severe gout.
To cite this abstract in AMA style:
Khanna P, Strand V, Singhal A, Baraf H, Azeem R, DeHaan W, Leung S, Santin-Janin H, Falk A, Kivitz A. Impact of Anti-drug Antibodies on the Efficacy of SEL-212 in Patients with Chronic Gout Refractory to Conventional Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-anti-drug-antibodies-on-the-efficacy-of-sel-212-in-patients-with-chronic-gout-refractory-to-conventional-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-anti-drug-antibodies-on-the-efficacy-of-sel-212-in-patients-with-chronic-gout-refractory-to-conventional-therapy/