Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
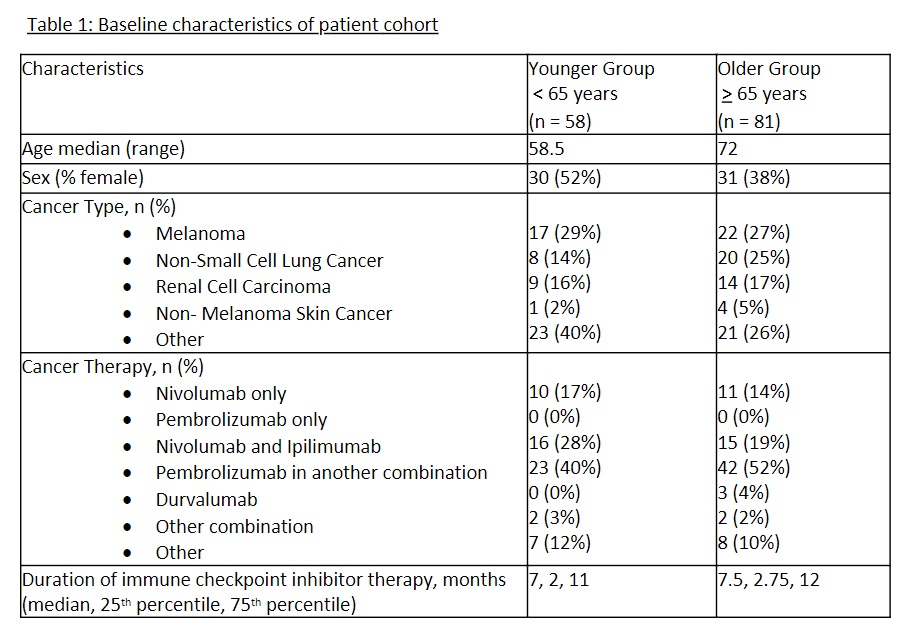
Background/Purpose: Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy. Their use is complicated by development of immune-related adverse effects (irAEs), including rheumatic irAEs (Rh-irAE). Aging is known to be associated with an increase in chronic inflammation, likely driven by age-related changes in inflammatory networks and proinflammatory pathways, referred to as “inflammaging”. Older age has been implicated with the development of more frequent and severe irAEs. In this study, we aim to examine whether older patients with Rh-irAEs develop more severe Rh-irAEs or greater number of irAEs compared to younger patients.
Methods: Adults who develop new Rh-irAEs after ICI exposure are prospectively followed at 9 academic sites across Canada as part of the CanRIO prospective cohort. We compared the severity and number of irAE between patients ≥65 years and < 65 years, using logistic regression and Poisson regression, respectively. As part of secondary analysis, we compared the immunosuppression and cancer treatment received in both groups.
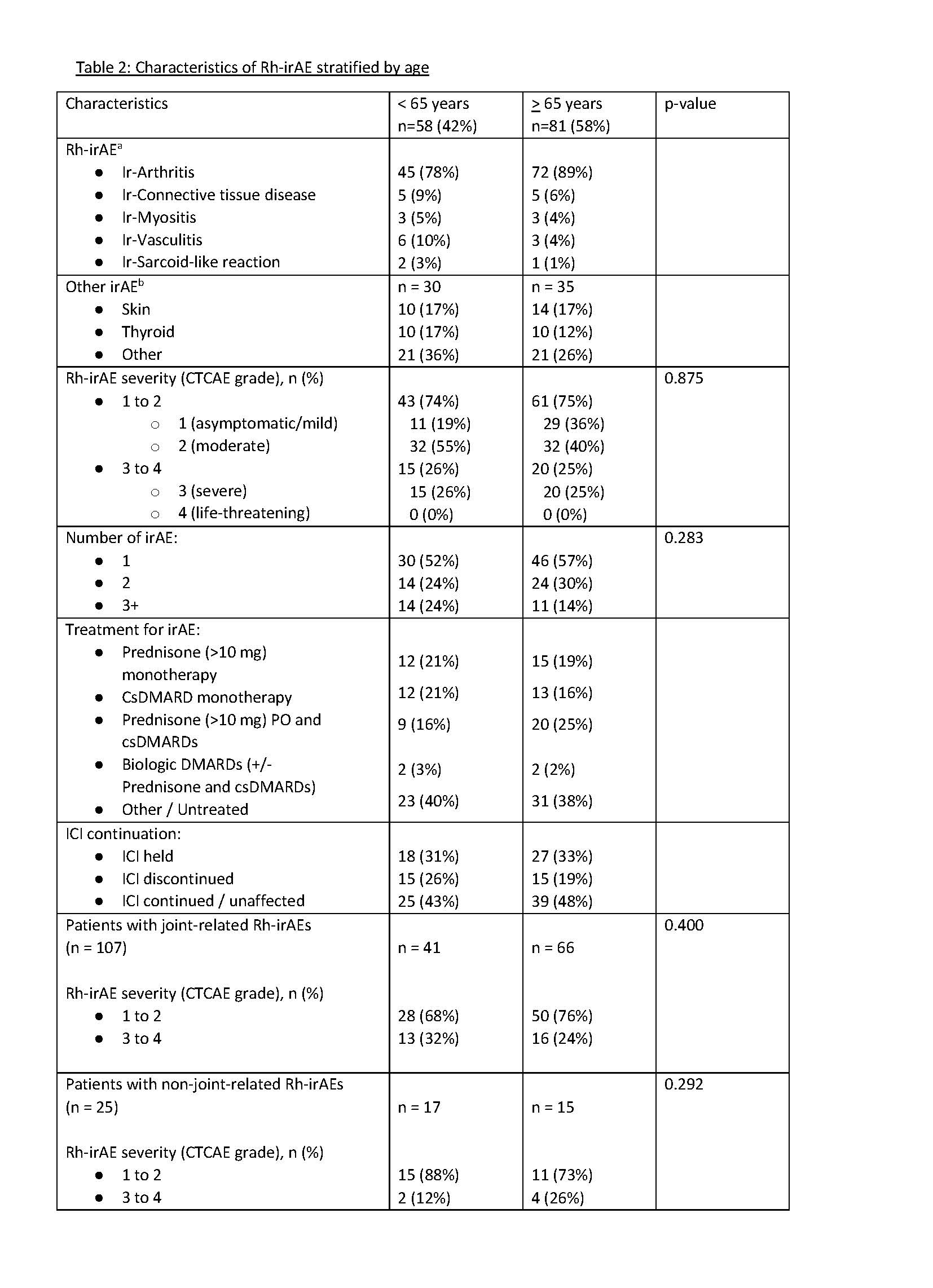
Results: 139 Patients with de novo Rh-irAEs recruited between Jan 2020 and March 2023 were included, 58 in the “younger” ( < 65 years) and 81 in the “older” (≥65 years) group (Table 1).There was no significant difference in severity of Rh-irAE (p =0.86) or number of irAEs in each group (p = 0.283) (Table 2). Treatment of irAEs was similar between the two groups, with most patients receiving prednisone monotherapy, conventional synthetic DMARD (csDMARD) monotherapy, or combinations of prednisone/csDMARDs. Biologic DMARDs were infrequently used in both groups. ICI use was similar in both groups. There was a trend towards more severe joint-related Rh-irAEs in the younger group (32% vs 24%, p-value = 0.400), and more severe non-joint related Rh-irAEs (12% vs. 26%, p-value = 0.292) in the older group.
Conclusion: There is growing literature on the role of aging and autoimmunity, but limited data on the relationship between aging and autoimmune reactions, especially after exposure to ICI. In this prospective cohort study, we found similar numbers of overall irAE and severity of Rh-irAE in older and younger patients and similar treatment in both groups. As the role of immunotherapy continues to expand, further investigations in this area can provide insight on whether age-related changes in the immune system influence the development, severity, and clinical course of irAE, which may impact patient counselling and underlying cancer therapy.
To cite this abstract in AMA style:
Li J, Hudson M, Ye C, Roberts J, Fifi-Mah A, Choi M, Hoa S, Appleton T, Pope J, Maltez N, Gonzalez Arreola L, Obrzut A, Jamal S. Impact of Aging on Rheumatic Immune-related Adverse Events Secondary to Immune Checkpoint Inhibitors: Experience from the Canadian Research Group of Rheumatology in Immuno-Oncology (CanRIO) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-aging-on-rheumatic-immune-related-adverse-events-secondary-to-immune-checkpoint-inhibitors-experience-from-the-canadian-research-group-of-rheumatology-in-immuno-oncology-canrio/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-aging-on-rheumatic-immune-related-adverse-events-secondary-to-immune-checkpoint-inhibitors-experience-from-the-canadian-research-group-of-rheumatology-in-immuno-oncology-canrio/