Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To evaluate health-related quality of life (HRQoL) in patients with active lupus nephritis (LN) at baseline and 12 months after treatment in relationship to the patients’ renal response.
Methods: GLADEL 2.0 is an observational prevalent and incident cohort initiated in 2019. Forty-four centers from 10 Latin-American countries enrolled patients ≥18 years of age who fulfilled the 1982/1997 ACR and/or the 2012 SLICC classification criteria for SLE. These patients were from four different groups according to the presence or absence of LN. For this analysis, patients in Group II (prevalent inactive LN), III (prevalent active LN), IV (incident LN, onset < 3 months), and follow-up data at 12 months were included. Demographic, clinical manifestations, treatments, disease activity (SLEDAI-2k) and damage SLICC/ACR Damage Index (SDI) were examined. At baseline, HRQoL was assessed with the LupusQoL and compared by the presence of active or inactive LN. At 12 months, LupusQoL was applied to the active LN patients as a function of their renal response. Partial and complete responses were defined according to EULAR/KDIGO: Complete Clinical Response (CCR): urinary protein-to-creatinine ratio (UPCR) < 0.5 g/g; Partial Clinical Response Criteria (PRC): ≥50% reduction in UPCR and No Response (NR): < 50% reduction in proteinuria. A descriptive analysis was performed.
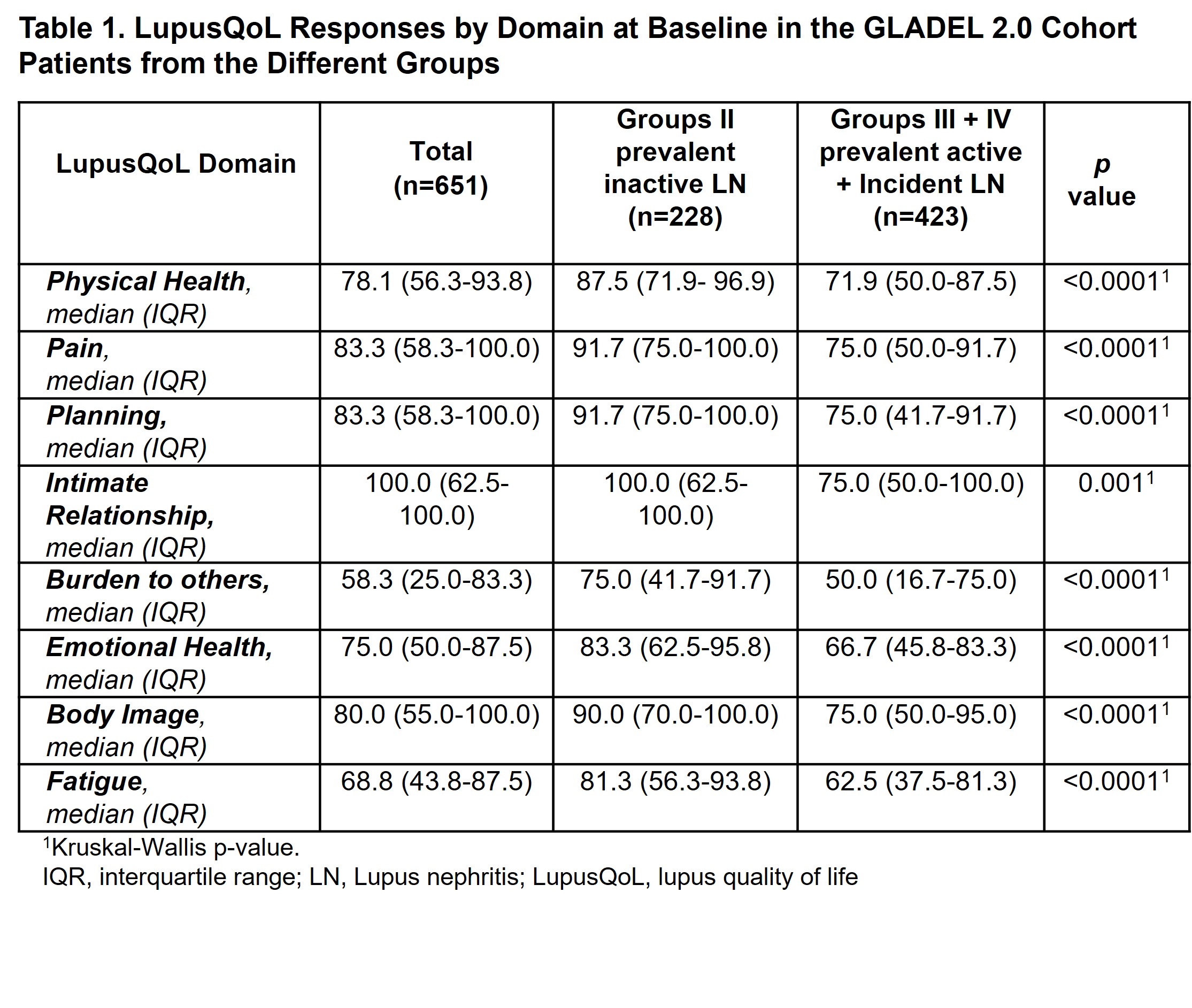
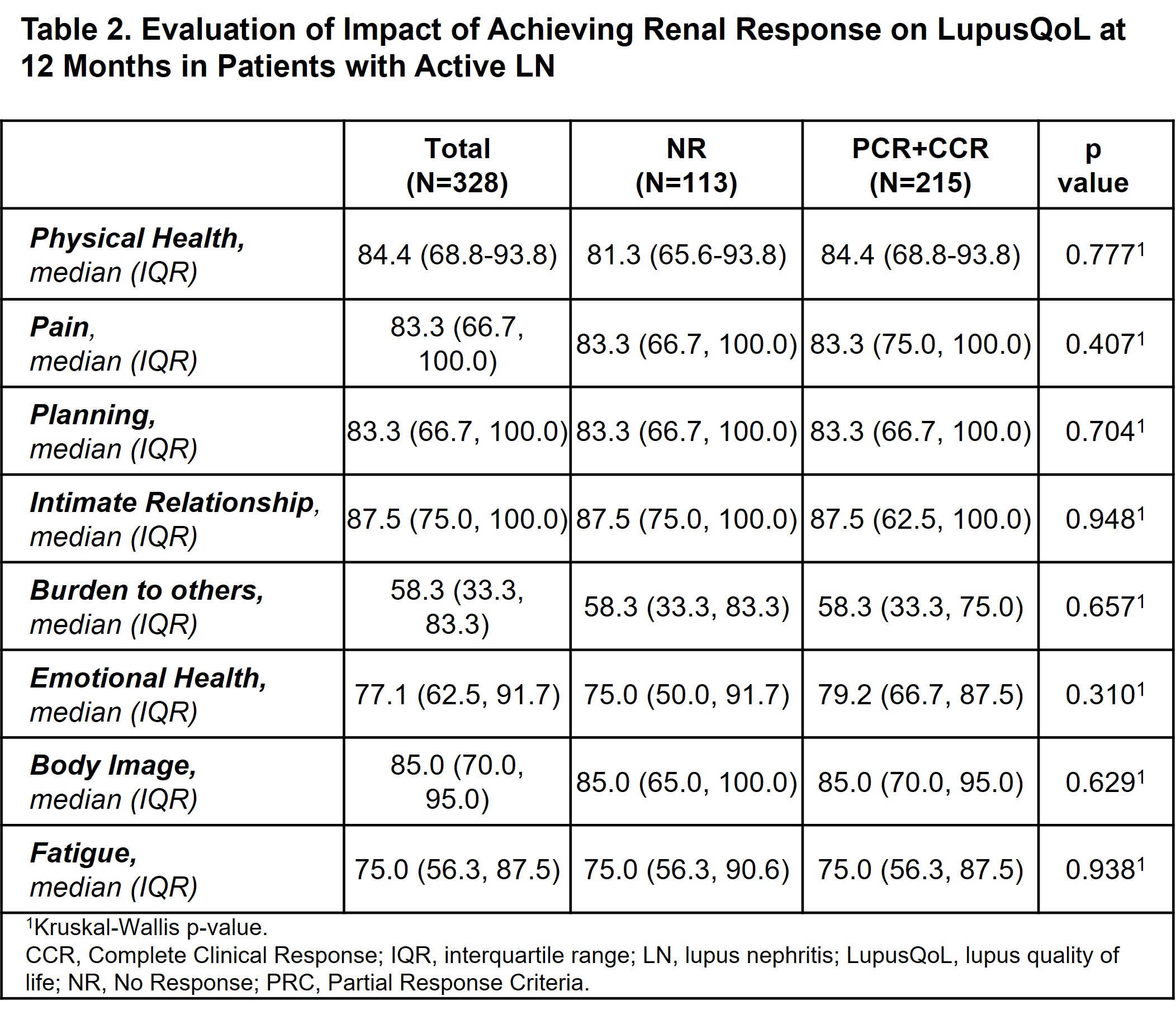
Results: Of the 1081 patients included in the cohort, 651 patients with LN were evaluated (423 with active and 228 with inactive disease). The active LN patients were predominantly women [569 (87.4%)], younger at cohort entry, of lower socioeconomic status, exhibited higher levels of unemployment, and had a higher SLEDAI than patients with inactive LN. As to the baseline LupusQol, it was found to be worse in patients with active LN in all domains (Table 1). At 12 months, however, no differences were found between those patients who achieved complete/partial renal response versus those who did not (Table 2).
Conclusion: At baseline, active LN patients showed a worse QoL compared to those with inactive LN. However, at 12 months no differences were found between patients who achieve or did not achieve a renal response. Future analyses with a larger number of follow-up patients would be necessary to provide conclusive data.
To cite this abstract in AMA style:
Nieto R, Quintana R, Ávila D, Serrano Morales R, Harvey G, Hernandez L, Roberts K, Meras N, Otaduy C, Novatti E, Arturi V, Palacios Santillan E, Kisluk B, González Lucero L, Kerzberg E, Pérez N, Pisoni C, Pirruccio P, Crespo M, Montandon A, Gasparin A, Duarte A, Alves Alvino L, Bonfa E, Figuereido Neves E, De Oliveira Martins L, Guerra I, Mimica Davet M, De La Hoz Rueda L, Cadena Bonfanti A, Rivera R, Coral Alvarado P, Fredy Jaramillo J, Martínez J, Moreno M, Sánchez Briones R, Pérez Cristóbal M, Martin Nares E, Juárez-Vicuña Y, González Bello Y, González O, Aguilar Rivera L, Duarte M, Langjarth P, Pérez Medina W, Calvo A, Polanco T, Pizzarossa C, Silveira G, Reategui C, Alarcon G, Sbarigia U, Zazzetti F, Orillion A, Pons-Estel G, Pons-Estel B. Impact of Active Lupus Nephritis on the Quality of Life of Patients from a Latin American Lupus Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-active-lupus-nephritis-on-the-quality-of-life-of-patients-from-a-latin-american-lupus-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-active-lupus-nephritis-on-the-quality-of-life-of-patients-from-a-latin-american-lupus-cohort/