Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Small vessel vasculitis mainly includes ANCA-associated vasculitis (AAV) and cryoglobulinemic vasculitis (CV), leading to significant organ damage. Rituximab (RTX), an anti-CD20 monoclonal antibody, has improved the prognosis by targeting B cells1. Anti-RTX antibodies (ARA) are detrimental in primary membranous glomerulonephritis2 and rheumatoid arthritis but their impact in small vessel vasculitis remains unclear. We aimed to evaluate the impact of ARAs on disease progression, treatment response and prognosis in patients with AAV and CV.
Methods: We retrospectively identified patients with AAV or CV who developed ARA through two immunology laboratories and the French Vasculitis Study Group. ARA positivity was defined by an undetectable serum RTX level with high anti-RTX antibody titer ( >20%). We evaluated demographic, clinical, and laboratory data; CD19+ B-cell counts at 6 months; and outcomes including relapse or refractory disease. We also analyzed two control cohorts (one for AAV, one for CV) to assess risk factors for immunization.
Results: Thirty-six patients with ARA-positive vasculitis were included (median age 58 IQR [42-69]; 75% female), including 19 CV (all associated with Sjögren’s syndrome) and 17 AAV. The most common organ involvement was skin (59%), peripheral nerve (42%), and kidney (41%). Patients received a median of five RTX infusions prior to ARA detection, with a median interval of 11 months (IQR [5-25]) from RTX initiation. Notably, 38% experienced serum sickness-like reactions during infusions prior to ARA identification. CD19+ B cells were detectable in 91% of patients at the time of ARA detection, and 91% had active disease at that time. After ARA detection, 27 patients (75%) required a change in therapy, with obinutuzumab being the most common alternative (50%), while others received cyclophosphamide, belimumab or ofatumumab. Clinical remission was achieved in 96% of patients, with 92% achieving B-cell depletion after switching (Figure 1). We next compared baseline and follow-up data between patients and controls (Table 1). In the CV group, variables independently associated with the detection of ARA were Sjogren’s syndrome (adjusted OR 31.2; 95%CI 3.4-268; p=0.001), age ≤40 years (aOR 9.21; 95%CI 1.29–65.8; p=0.027), reactions during RTX infusions (aOR 25.6; 95%CI 3.30–198; p=0.002) and the use of RTX regimen 1 gram on days 1 and 15 (aOR 10.1; 95%CI 2.33–43.8; p=0.002). In the AAV group, variables independently associated with the detection of ARA were microscopic polyangiitis (aOR 3.7; 95%CI 1.21–10.0; p=0.021), renal involvement (aOR 3.36; 95%CI 1.01–11.2; p=0.048) and mononeuritis multiplex (aOR 3.58; 95% CI [1.19–10.8]; p=0.023), and the use of RTX regimen 1 gram on days 1 and 15 (aOR 4.87; 95%CI 1.59-14.9; p=0.006) (Table 2).
Conclusion: In conclusion, ARA detection is associated with younger age, Sjogren’s syndrome and infusion-related reactions in cryolgobulinemia vasculitis, and with microscopic polyangiitis, renal involvement, and mononeuritis multiplex in AAV, with the RTX regimen of 1 gram on days 1 and 15 being associated with ARA detection in both vasculitis. Monitoring and switching to obinutuzumab may improve outcomes.
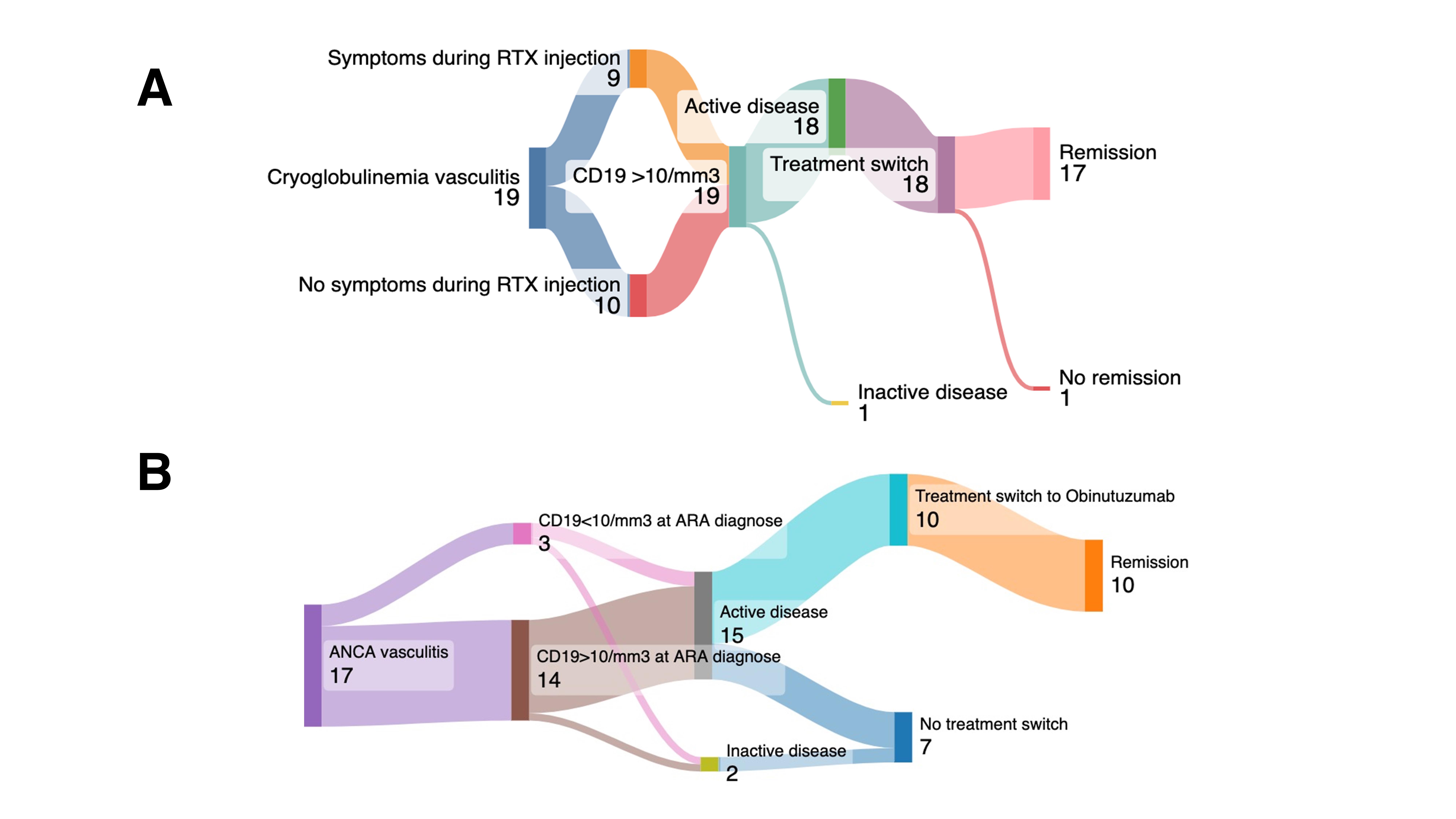 Figure 1. Sankey Diagram of Clinical Features, CD19 Reconstitution, and Treatment Outcomes in Cryoglobulinemic and ANCA-Associated Vasculitis Cohorts. A. Cyroglobulinemic vasculitis ARA’s cohort: Among 19 patients with cryoglobulinemic vasculitis who developed anti-rituximab antibodies (ARA), half experienced infusion-related symptoms during rituximab (RTX) administration. All 19 showed CD19+ reconstitution (>10 cells/mm³) at the time of ARA detection; 18 had active disease and were switched to an alternative therapy, leading to remission in 17 cases (1 remained with persistent disease). B. ANCA-associated vasculitis patients with ARA, 14 exhibited CD19+ reconstitution (>10 cells/mm³) and 3 maintained depletion ( < 10 cells/mm³). Fifteen had active disease, of whom 10 were switched to obinutuzumab—achieving remission in all—and 7 continued their previous regimen without switch (2 were inactive at ARA diagnosis).
Figure 1. Sankey Diagram of Clinical Features, CD19 Reconstitution, and Treatment Outcomes in Cryoglobulinemic and ANCA-Associated Vasculitis Cohorts. A. Cyroglobulinemic vasculitis ARA’s cohort: Among 19 patients with cryoglobulinemic vasculitis who developed anti-rituximab antibodies (ARA), half experienced infusion-related symptoms during rituximab (RTX) administration. All 19 showed CD19+ reconstitution (>10 cells/mm³) at the time of ARA detection; 18 had active disease and were switched to an alternative therapy, leading to remission in 17 cases (1 remained with persistent disease). B. ANCA-associated vasculitis patients with ARA, 14 exhibited CD19+ reconstitution (>10 cells/mm³) and 3 maintained depletion ( < 10 cells/mm³). Fifteen had active disease, of whom 10 were switched to obinutuzumab—achieving remission in all—and 7 continued their previous regimen without switch (2 were inactive at ARA diagnosis).
.jpg) Table 1 Baseline features, treatment regimens and follow-up outcomes of the study cohorts. A. Comparison between CV patients without anti-rituximab antibodies (CryoVas cohort, N = 321) versus those with detectable anti-rituximab antibodies (ARA cryoglobulinemia, N = 19). B. Comparison between patients with ANCA-associated ARA (N = 202) versus ARA-ANCA patients with detectable anti-rituximab antibodies (N = 17).
Table 1 Baseline features, treatment regimens and follow-up outcomes of the study cohorts. A. Comparison between CV patients without anti-rituximab antibodies (CryoVas cohort, N = 321) versus those with detectable anti-rituximab antibodies (ARA cryoglobulinemia, N = 19). B. Comparison between patients with ANCA-associated ARA (N = 202) versus ARA-ANCA patients with detectable anti-rituximab antibodies (N = 17).
.jpg) Table 2. Univariable and multivariable predictors of anti-rituximab antibody development in small-vessel vasculitis. A. illustrates the associations between key clinical factors and anti-rituximab antibody (ARA) positivity in cryoglobulinemic vasculitis (CV), using both univariable and multivariable Firth‐penalized logistic regression. B. presents the same analyses for ANCA-associated vasculitis (AAV). Odds ratios and 95% confidence intervals were estimated via the logistf package; multivariable models adjust for all variables shown in each panel.
Table 2. Univariable and multivariable predictors of anti-rituximab antibody development in small-vessel vasculitis. A. illustrates the associations between key clinical factors and anti-rituximab antibody (ARA) positivity in cryoglobulinemic vasculitis (CV), using both univariable and multivariable Firth‐penalized logistic regression. B. presents the same analyses for ANCA-associated vasculitis (AAV). Odds ratios and 95% confidence intervals were estimated via the logistf package; multivariable models adjust for all variables shown in each panel.
To cite this abstract in AMA style:
Khellaf L, Seror R, ricordi c, chhun s, gleizes a, lobbes h, pouchelon c, Besse m, Kahn J, revuz s, Cumin M, Titeca-Beauport D, Papo M, Rémy P, Desprets m, garo f, Ronsin C, Hacein-Bey-Abina S, Servettaz A, Patrice C, Puéchal X, Terrier B. Impact and risk factors of anti-rituximab antibodies in small-vessel vasculitis: a multicenter retrospective study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-and-risk-factors-of-anti-rituximab-antibodies-in-small-vessel-vasculitis-a-multicenter-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-and-risk-factors-of-anti-rituximab-antibodies-in-small-vessel-vasculitis-a-multicenter-retrospective-study/
