Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Given an increased risk of COVID-19 in patients with autoimmune conditions, we must better understand the immunogenicity and safety of SARS-CoV-2 vaccines in people living with rheumatic disease. We conducted the Covid VaccinE Response (COVER) trial, a multicenter, randomized controlled trial to assess the response to the SARS-CoV-2 booster in rheumatology patients on immunomodulatory therapies. A primary goal was to assess whether patients randomized to hold therapy for 2 weeks after a booster dose had improved vaccine response compared to those who continued therapy.
Methods: The COVER trial enrolled patients with rheumatoid arthritis (RA) or spondylarthritis (SpA) and was conducted in the Excellence Network in RheumatoloGY (ENRGY), a large practice-based research network (PBRN) of US rheumatologists. Participants were randomized to continue or hold current medication, abatacept (ABA) or tumor necrosis factor inhibitors (TNFi), for 2 weeks following a booster dose of COVID-19 vaccine. All patients had received primary vaccination against COVID-19 with an mRNA vaccine. LabCorp Cov2Quant IgG™ Spike assay measured levels of anti-Receptor Binding Domain (RBD) IgG antibodies prior to and following the booster dose. For each medication group (ABA or TNFi), we looked at the mean log-transformed difference pre- and post-booster and used linear regression to compare the two groups with respect to change in COVID-19 RBD antibody levels, adjusting for age, sex, BMI, methotrexate use, and number of prior vaccine doses.
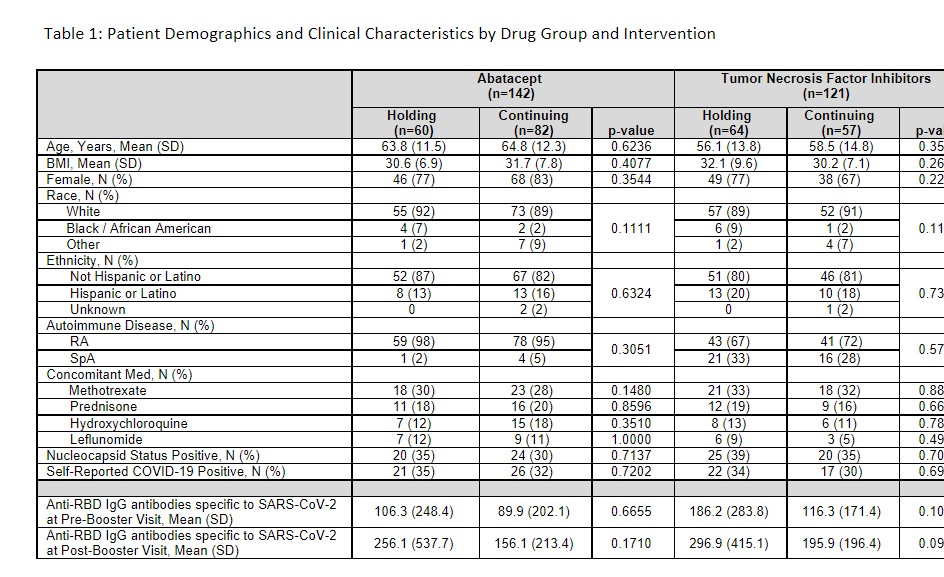
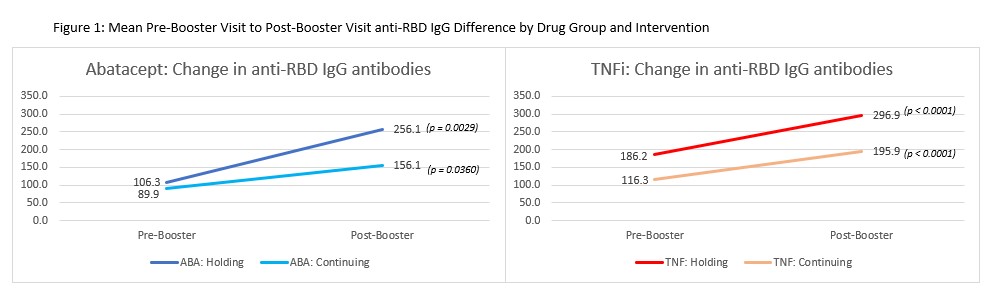
Results: Within both the abatacept (n=142) and TNFi (n=121) medication groups, there were no significant baseline differences in demographics or clinical characteristics (Table 1). Combining both groups, the population was 76% female, 92% white, 17% Hispanic, with mean (SD) age of 61.1 (13.5) years, and mean (SD) BMI of 31.2 (8.2) kg/m2. The ABA group was significantly older than the TNF group: 64.8 (11.7) vs 57.1 (14.3), p< 0.0001). Following the COVID booster vaccine, RBD antibody titers increased significantly in both ABA (p=0.0029 for holding; p=0.03604 for continuing) and TNF (p < 0.0001 for both arms) patients (Figure 1). Patients who continued their ABA had a numerically lower increase in anti-RBD antibody titers compared to those who held it for two weeks. However, in linear regression analysis, the mean increase in the holding arm compared to the continuing arm was not significantly different for either drug group in unadjusted analysis or after adjusting for demographic and clinical factors (a significant effect was seen for BMI (β=0.04, p=0.03) in the ABA analysis and MTX use (β=0.63, p=0.02) in TNFs). There was no significant interaction in the effect of holding or continuing medication between the ABA and TNFi groups (interaction p-value = 0.55).
Conclusion: RA and SpA patients receiving abatacept and tumor necrosis factor inhibitors had a significant increase in antibody response after receiving a COVID-19 booster. There was a weak and non-significant trend toward improved response in the group that held ABA for up to 2 weeks post-booster that was not significant. Based on these results, briefly interrupting ABA or TNFi at the time of COVID boosting may not appear warranted.
To cite this abstract in AMA style:
Mudano A, Cutter G, Mikuls T, Thiele G, Holladay E, Withrop K, Law M, Hamilton B, Bastidas M, Zikry M, Chun K, George M, Curtis J. Immunomodulatory Treatment and Autoimmune Patient Responses to COVID-19 Booster Shots: Results from the Covid-19 VaccinE Response in Rheumatology Patients (COVER) Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immunomodulatory-treatment-and-autoimmune-patient-responses-to-covid-19-booster-shots-results-from-the-covid-19-vaccine-response-in-rheumatology-patients-cover-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunomodulatory-treatment-and-autoimmune-patient-responses-to-covid-19-booster-shots-results-from-the-covid-19-vaccine-response-in-rheumatology-patients-cover-study/