Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: To study in daily practice the risk of immunogenicity of patients treated with rituximab (RTX) biosimilar GP2013 for their chronic inflammatory rheumatic disorder.
Methods: A prospective routine care study was carried out between September 2018 and May 2021 in the Rheumatology department of Cochin Hospital. We consecutively included patients treated with the biosimilar RTX GP2013, systematically used in the department since March 2018. Samples were taken before each infusion to quantify anti-RTX antibodies (ADAbs) and serum RTX trough levels by ELISA (Lisa Tracker Duo Rituximab, LTR005, Theradiag).
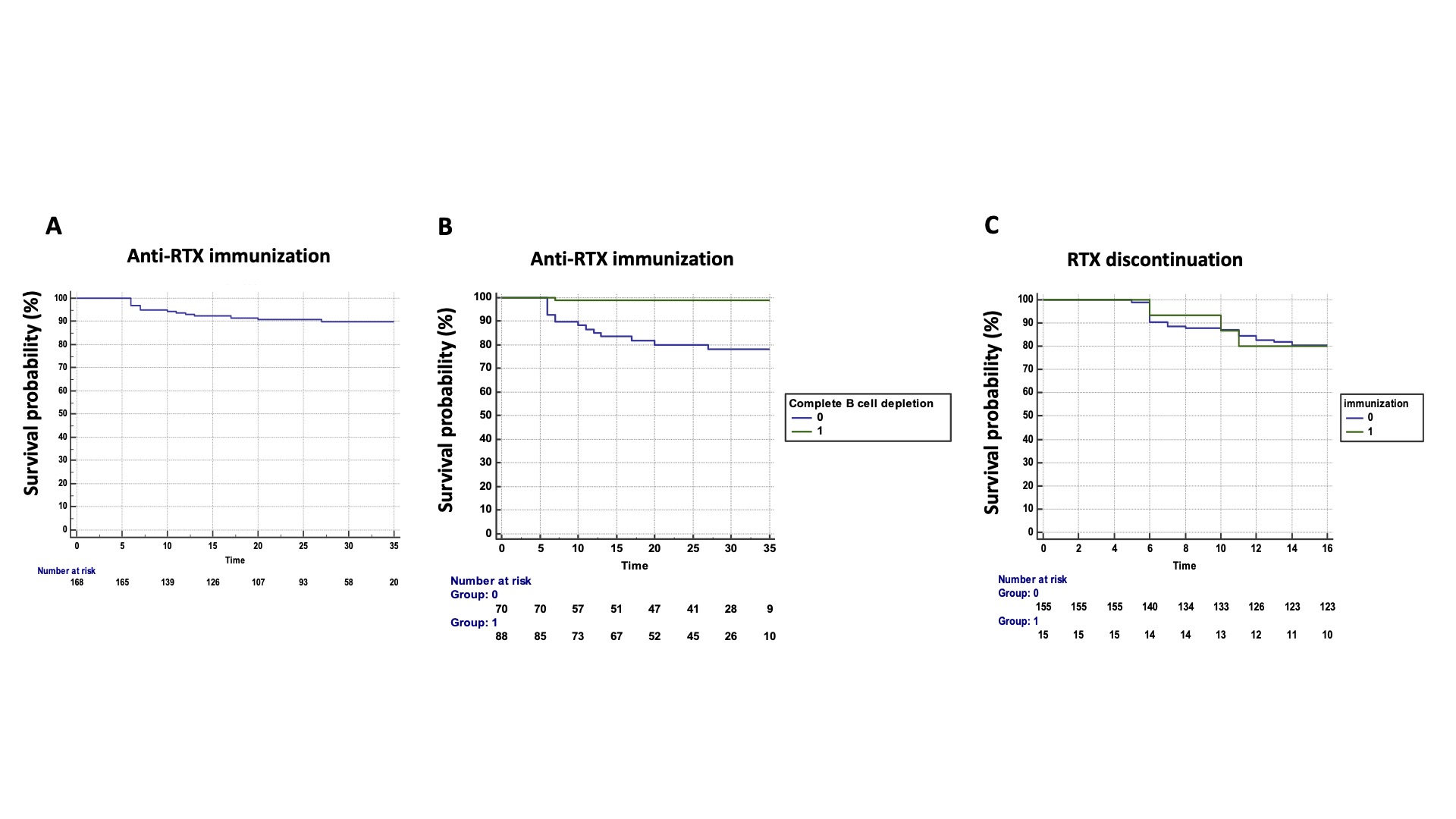
Results: We included 170 consecutive patients treated with GP2013 (134 women, 79%) with a mean age of 59±12 years and a mean disease duration of 18±11 years. Among these 170 patients, 114 (67%) had RA and 56 had another disease (17 systemic sclerosis (SSc), 13 mixed connective tissue disease (MCTD), 7 undifferentiated polyarthritis, 5 systemic lupus (SLE), 5 inflammatory myopathies (MI), 5 overlap syndrome, and 4 primary Sjögren syndrome). 146 patients (86%) were receiving associated disease-modifying therapy (DMARD), mainly methotrexate (MTX, 109/146 patients, 75%). 129 patients (76%) were in maintenance therapy with originator RTX (RTX exposure: 77±47 months and cumulative dose of RTX: 8±6g) before the switch to GP2013 in March 2018. Originator RTX was not re-established during the entire treatment period. The other 41 patients (24%) treated with GP2013 were naïve of originator RTX. During a mean follow-up of 25±10 months, 15 patients (8%), developed ADAbs, corresponding to a rate of 4 for 100 patient years (Figure 1A). Among the 15 patients with ADAbs, 9 had RA, 3 MCTD, 2 SSc and 1 SLE. The mean time to ADAbs detection was 11±6 months (range: 6-27 months). ADAb concentrations ranged from 6 to >100 ng/mL, with 3 patients with high ADAb concentrations >100 ng/mL. Patients with ADAbs had undetectable serum RTX trough levels. The frequency of immunization was higher among RTX-naïve patients (8/41, 19.5%) than in patients switching from originator RTX (7/129, 5%, p=0.004). Age, gender, disease duration, concomitant treatment with MTX, a body mass index >30 and the underlying disease were not predictive of immunization. Conversely, complete B cell depletion (CD19 < 18) was identified as protective of immunization (hazard ratio 0.04, 95% confidence interval, CI, 0.09-0.33, p=0.003) (Figure 1B). Moreover, ADAb concentrations correlated with CD19 counts (r=0.20, p=0.021). The presence of anti-RTX antibodies was not associated with increased disease flares or RTX discontinuation (Figure 1C). Regarding the 3 patients with ADAb concentrations >100 ng/mL, 1 patient experienced a severe allergic reaction leading to treatment discontinuation and the two others required RTX dose escalation from 500 mg to 1 g to maintain treatment efficacy.
Conclusion: The immunogenicity of the biosimilar RTX GP2013 is a rare event associated with incomplete B cell depletion. Although development of anti-RTX antibodies had no impact on disease flares and treatment discontinuation, possible meaningful consequences may be observed in patients with high antibody levels.
To cite this abstract in AMA style:
Avouac J, Cougnaud-Murail R, Goulvestre C, Dumas S, Molto A, Miceli-Richard C, conort o, Batteux F, Allanore Y. Immunogenicity of Rituximab Biosimilar GP2013 in Chronic Inflammatory Rheumatic Disorders in Daily Clinical Practice [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immunogenicity-of-rituximab-biosimilar-gp2013-in-chronic-inflammatory-rheumatic-disorders-in-daily-clinical-practice/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-of-rituximab-biosimilar-gp2013-in-chronic-inflammatory-rheumatic-disorders-in-daily-clinical-practice/