Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: AIIRD patients may have a blunted immune response to the COVID-19 vaccines, but this is uncertain as these individuals were not included in clinical trials. To better understand the immunogenicity of the COVID-19 vaccines in this population and the effects of systemic IMT used to treat them, we quantified humoral responses to the SARS-CoV-2 spike protein in AIIRD and control patients. This is an updated report of the pilot study presented at ACRC 2021.
Methods: This prospective cohort study enrolled patients from a single-specialty practice in Charlotte NC who received the BNT162b2 (Pfizer), mRNA-1273 (Moderna), or Ad26COV2.S (Johnson & Johnson) vaccine from 3/17/21-10/1/21. Those with prior COVID-19 by self-report were excluded. We collected demographics, diagnoses, and IMT of AIIRD patients. Antibody (Ab) testing was performed at >14 days after completion of their vaccine series. SARS-CoV-2 IgG Ab levels to the S1 spike antigen RBD were measured using a semi-quantitative assay (Siemens Atellica; Quest). Associations between clinical characteristics and Ab responses were evaluated. Similar data were collected in a control group of patients with non-inflammatory disease who were not on IMT.
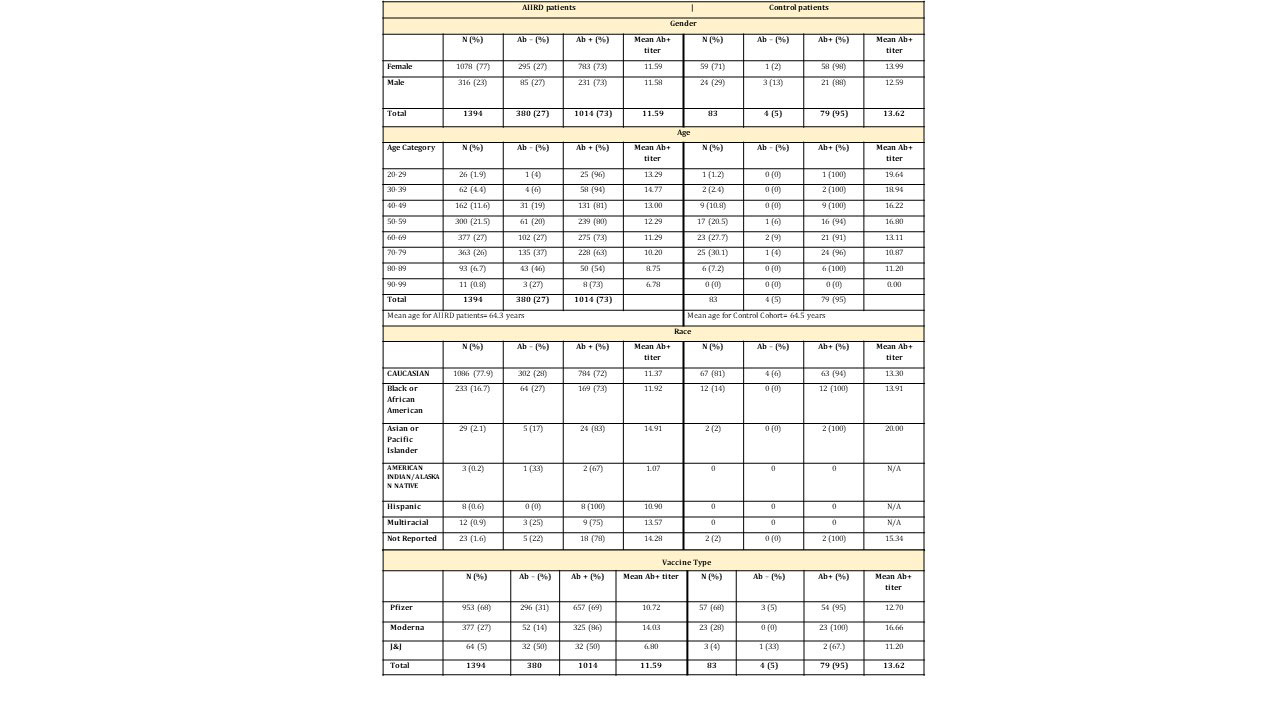
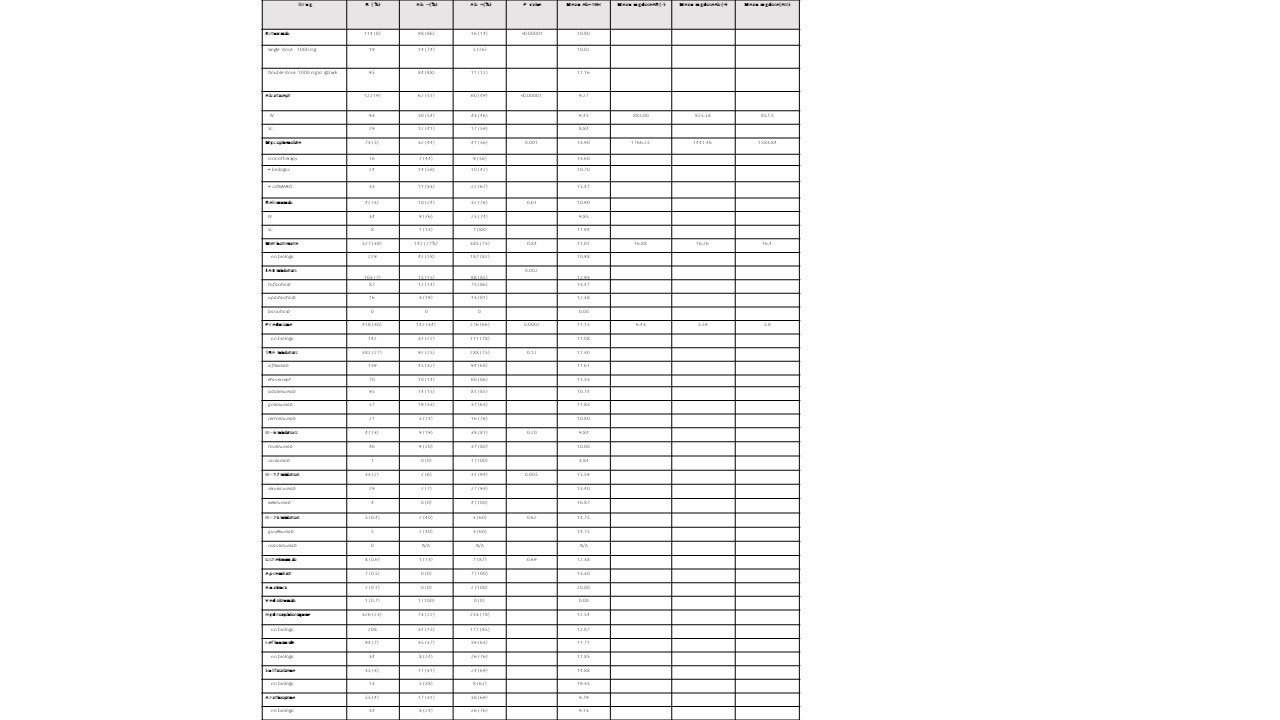
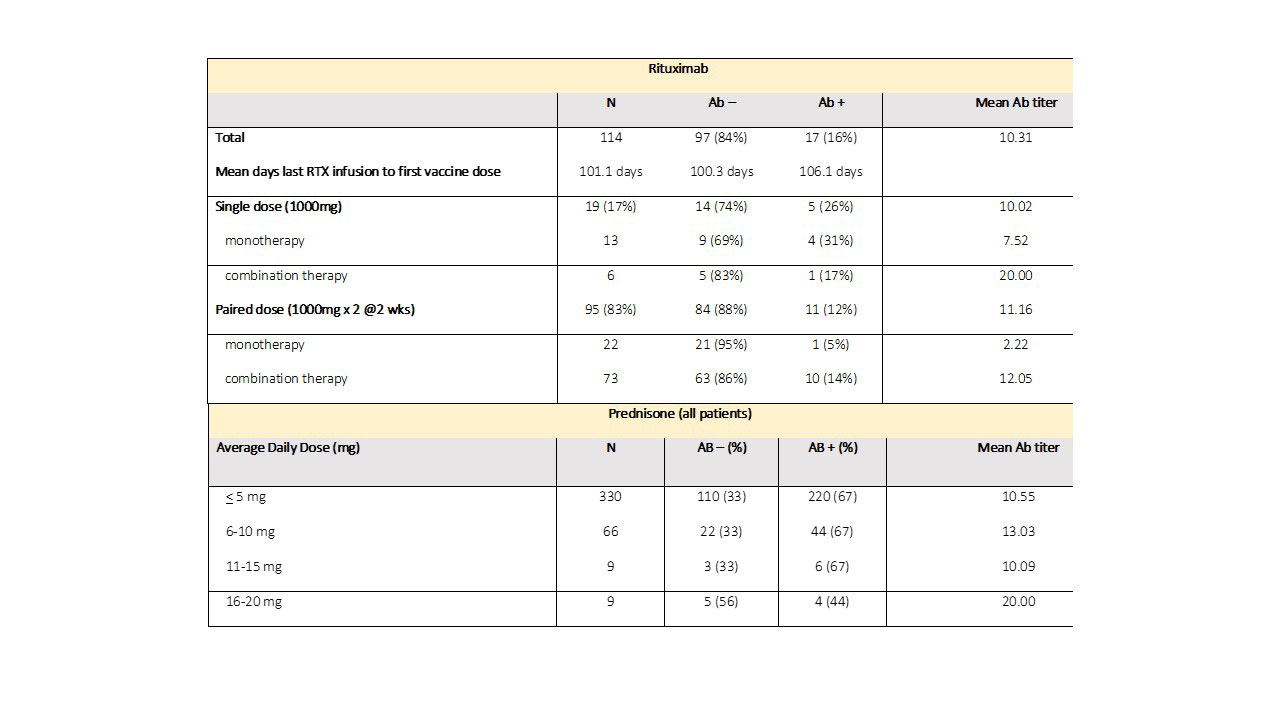
Results: Characteristics of 1394 AIIRD and 83 control patients are listed in Table 1. Overall, 73% of AIIRD patients developed an Ab response compared to 95% of controls (p< 0.001), with mean Ab titers of 11.59 (< 1 index) and 13.62, respectively (p< 0.001). There was no difference based on gender. The likelihood of an absent Ab response increased with age, and there was a decline in mean levels with aging in Ab+ patients. Treatment associations are listed in Table 2. Patients receiving rituximab were less likely to develop an Ab response (14%) and had a lower mean Ab titer (10.80) compared to the overall cohort. Dosing appeared to affect response rates with a single dose (1000 mg) Ab+ rate of 26% compared to a paired dose (1000 mg x2) rate of 12% (Table 3). Patients receiving abatacept were also less likely to develop an Ab response (49%). Detectable Abs were seen in most patients on JAKi (85%), belimumab (76%), TNFi (75%), IL-17i (94%), IL-6i (81%), and IL-12/23i (87%). Without concomitant targeted therapy, Ab+ rates with SSZ (69%), AZA (69%), LEF (63%), and MMF (56%) were decreased compared to MTX (82%) and HCQ (78%). 78% of patients receiving prednisone without biologics were Ab+ (average daily dose 5.8 mg). Overall dose effect is shown in Table 3.
Conclusion: AIIRD patients developed anti-SARS-CoV-2 RBD Abs at a decreased rate (73%) compared to controls (95%) with a lesser mean titer. Overall Ab response rates and titers were age dependent. Patients receiving rituximab and abatacept were less likely to develop an Ab response with the former having a dose effect. Among csDMARDs, response rates were decreased in patients receiving SSZ, AZA, LEF, and MMF. Low dose prednisone without biologics did not appear to influence Ab+ rates. Limitations of this study include its single center, non-randomized design and lack of residual confounder measures. Additional measures of humoral immune response and T cell function are also needed. However, this real-world study may provide further guidance for COVID-19 vaccination in patients with AIIRD on IMT.
To cite this abstract in AMA style:
Lam G, Laster A, Gladue H, Kashif A, Siceloff E, Lackey V, Robertson C, Toci A, McCarter M, Calabrese L. Immunogenicity of COVID-19 Vaccines in Patients with Autoimmune and Inflammatory Rheumatic Diseases (AIIRD) on Immunomodulatory Therapies (IMT): An Updated Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immunogenicity-of-covid-19-vaccines-in-patients-with-autoimmune-and-inflammatory-rheumatic-diseases-aiird-on-immunomodulatory-therapies-imt-an-updated-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-of-covid-19-vaccines-in-patients-with-autoimmune-and-inflammatory-rheumatic-diseases-aiird-on-immunomodulatory-therapies-imt-an-updated-cohort-study/