Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Immunogenicity of anti-TNF therapies in Patients with inflammatory rheumatic Diseases and secondary failure: a multicentre study of 570 patients.
Background/Purpose: The treatment of rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA) has been greatly improved by the introduction of TNF inhibitors (TNFi). However, a proportion of patients receiving these biologic therapies still persist with active disease, either because the treatment fails to initiate a response (primary failure), or initial responsiveness gives rise to non-response (secondary failure). One of the potential reasons for secondary failure is the development of anti-drug antibodies. The primary objective of the study was to evaluate the prevalence of anti-drug antibodies in patients with RA or SpA who had a secondary failure to anti-TNF therapy. Secondary objectives included the evaluation of the relationship between anti-TNF concentration, presence of antibodies against anti-TNF, and clinical response. The study also aimed to evaluate the relationship between presence of antibodies against anti-TNF and concomitant use of DMARDs, and the correlation of antidrug antibody positivity and RF and anti-CCP status in patients with RA.
Methods: Cross-sectional, observational study in 45 rheumatology centers across Spain between November 2012 and July 2014. Patients were eligible for inclusion if they were aged ≥18 years, had active RA or SpA (including PsA), and had received and complied with anti-TNF [etanercept (ETN), infliximab (INF) or adalimumab (ADA))] treatment. Patients with secondary failure to anti-TNF at the time of the study visit were consecutively included. Secondary failure was defined as: good response during at least three months (Disease Activity Score (DAS28) ≤3.2, Ankylosing Spondylitis Disease Activity Score [ASDAS] ≤ 2.1 or Bath Ankylosing Spondylitis-Disease Activity Index [BASDAI] < 4) to the current anti-TNF and current moderate-to-high disease activity (DAS28 >3.2 or ASDAS >2.1 or BASDAI ≥ 4). Concomitant non-biologic DMARDs during the observation period was permitted. Trough serum anti-TNF concentration and serum anti-drug antibody concentration were measured with two-site enzyme-linked immunosorbent assay (ELISA) using the Promonitor® (Proteomika) kits. All assays were performed in a central reference laboratory. Tests were considered positive when serum drug levels and anti-drug antibody concentrations exceeded stated limits (INF: 50μg/ml and 37 AU/ml; ADA 4 μg/ml and 35 AU/ml; ETN: 52 μg/ml and 138 AU/ml, respectively). Demographic and clinical characteristics were summarised using descriptive statistics. Quantitative variables were analysed using measurements of central tendency and of dispersion. Qualitative variables were defined according to their absolute and relative frequencies. Student’s t-test, Mann-Whitney-U test or Kruskall Wallis H test were used to compare quantitative variables and Pearson’s chi-square or Fisher exact tests for qualitative variables.
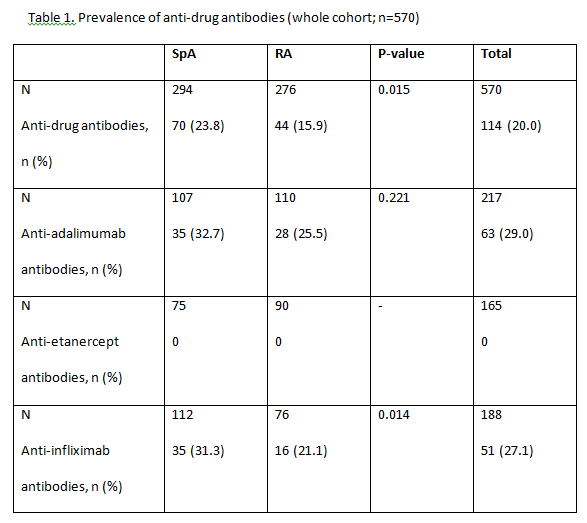
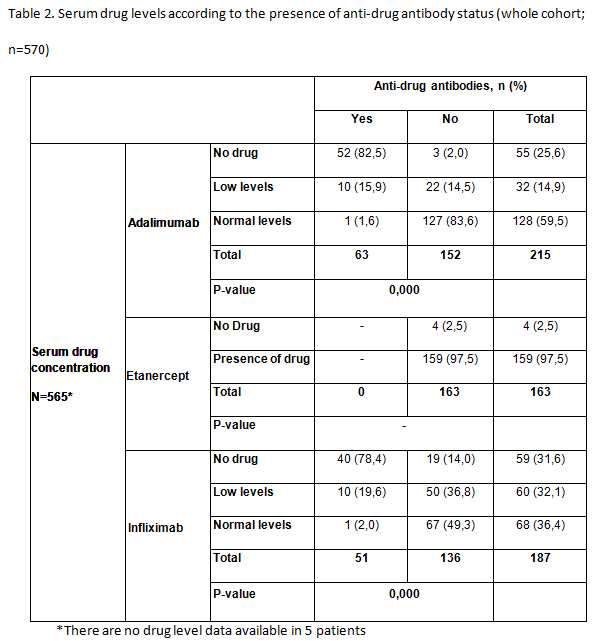
Results: A total of 583 patients with secondary failure were recruited into the study and 570 were considered evaluable (RA, n=276; SpA, n=294). Patient demographics and patient characteristics were significantly different between patients with RA and SpA at baseline. Patients with RA were majority female (80%), older and took longer to obtain good clinical disease control; more patients with RA than SpA were taking concomitant DMARDs (83 vs 47%), but approximately 75% of patients in both groups took concomitant methotrexate. 114/570 (20.0%) patients with secondary treatment failure developed anti-drug antibodies (Table 1). 51/188 (27.1%) patients were found to be positive for anti-INF antibodies and 63/217 (29.0%) patients for anti- ADA antibodies. Significantly more patients with SpA had anti-INF antibodies than those with RA (23% vs 15.9%; P=0.015). Antibodies were not detected in patients treated with ETN. In patients treated with ADA and INF, the majority of patients with no detectable drug levels also had anti-drug antibodies (Table 2). Low serum drug levels were detected mainly in patients with no anti-drug antibodies, though some patients with antibodies still had low but measurable levels of drug. Of the 114 patients who developed anti-drug antibodies, 81% of them had no detectable drug levels in their serum. The proportion of patients with anti- ADA and anti-INF antibodies was statistically lower in patients receiving concomitant DMARDs versus those on monotherapy (p<0.05). No statistically significant differences were observed between groups of patients with or without anti-drug antibodies, and positive levels of RF or anti-CCP antibodies for any biologic treatment for RA
Conclusion: In this observational study of patients diagnosed with RA or SpA, we found that antibodies against TNFi were present in 20% of patients (28% of those treated with monoclonal antibodies [63/217 (29%) treated with ADA; 51/188 (27%) treated with INF and in any patient treated with ETN] who developed secondary failure to TNFi. Most patients with antidrug antibodies had no detectable trough drug serum levels. The development of anti-drug antibodies is a a major contributor to secondary treatment failure in patients treated with monoclonal antibodies, immunogenicity could not explain a significant proportion of secondary failures in this population. Further investigations are needed to determine all possible causes of failure to anti-TNF therapy
To cite this abstract in AMA style:
Balsa A, Sanmarti R, Rosas J, Gomez Castro S, Cabez A, Martin V, Montoro M. Immunogenicity of Anti-TNF Therapies in Patients with Inflammatory Rheumatic Diseases and Secondary Failure: A Multicentre Study of 570 Patients [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/immunogenicity-of-anti-tnf-therapies-in-patients-with-inflammatory-rheumatic-diseases-and-secondary-failure-a-multicentre-study-of-570-patients/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-of-anti-tnf-therapies-in-patients-with-inflammatory-rheumatic-diseases-and-secondary-failure-a-multicentre-study-of-570-patients/