Session Information
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at increased risk of contracting herpes zoster (HZ) and treatment with JAnus Kinase inhibitors (JAKi) further adds on to that risk. A novel subunit vaccine against HZ (HZ/su; Shingrix) composed of VZV glycoprotein E (gE) and adjuvant system AS01B has recently been licensed. The HZ/su vaccine has been shown safe and effective in healthy elderly but data on patients with rheumatic disease are scarce. The objective of this study was to investigate the antibody responses to the HZ/su vaccine in RA patients treated with JAKi and controls.
Methods: RA patients at the rheumatology outpatient department, Skåne University Hospital, Lund, Sweden, fulfilling the ACR87 criteria and receiving JAKi as monotherapy or in combination with other disease modifying anti-rheumatic drugs (DMARDs) and individuals ³18 years without known rheumatic disease and not receiving immunosuppressive treatment for other conditions served as controls. All participants were immunized with two intramuscular doses of 50 mg HZ/su vaccine administered at least two months apart. Blood was sampled prior to first and 4-6 weeks after the second vaccine dose. ELISA using varicella zoster virus (VZV) glycoprotein E (gE) as the antigen was employed to measure anti-gE levels (1). Results are presented as optical density (OD). A positive antibody response was defined as a difference of ³1.2 in OD between the post- and pre-vaccination sera. The difference between ODs before and after vaccination among patients and controls separately were calculated using Wilcoxon signed-rank test. Chi2 test was used to compare % of individuals with positive antibody response between the two groups.
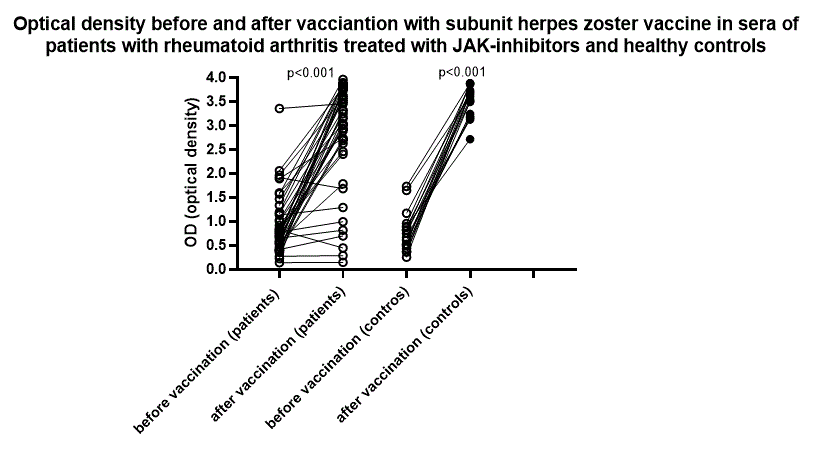
Results: Data from 40 RA patients (mean age 62.3 years; 77.5% female, mean disease duration 16 years) treated with JAKi and 20 controls (mean age 61.5 years and 70% female) were analysed. Compared to prevaccination sera, OD levels increased significantly in both patients and controls (p< 0.001). Mean changes in anti-gE antibody OD between post- and prevaccination sera were +1.8 and +2.6 for patients and controls respectively (Figure1). Positive humoral responses (delta OD³1.2) were observed in 75% of patients and 100% of controls (p=0.014). The majority of patients reported mild to moderate vaccine related side effects (pain and redness at the injection site, headache, fever for a few days) which resolved without any treatment. In total, 3 patients and 0 controls withdrew from the study prior to the second vaccine dose, 2 of these due to adverse events (diarrhoea, joint and muscle pain) and are not included in these preliminary results. No patient experienced worsening or flare of RA. No case of HZ has been reported yet (up to 15 months after start of enrolment in the study).
Conclusion: Conclusion: These preliminary results show satisfactory serological responses and acceptable tolerability of two doses HZ/su vaccine in RA patients treated with JAKi known to be at a particularly increased risk of HZ.
Reference
- Thomsson E, Persson L, Grahn A, et al. Recombinant glycoprotein E produced in mammalian cells in large-scale as an antigen for varicella-zoster-virus serology. J Virol Methods. 2011;175(1):53‐59.
To cite this abstract in AMA style:
Källmark H, Gullstrand B, Nagel J, Einarsson J, Jönsson G, Kahn F, Kahn R, Bangtsson A, Bergström T, Kapetanovic M. Immunogenicity of Adjuvanted Herpes Zoster Subunit Vaccine in Rheumatoid Arthritis Patients Treated with Janus Kinase Inhibitors and Controls: Preliminary Results [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/immunogenicity-of-adjuvanted-herpes-zoster-subunit-vaccine-in-rheumatoid-arthritis-patients-treated-with-janus-kinase-inhibitors-and-controls-preliminary-results/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-of-adjuvanted-herpes-zoster-subunit-vaccine-in-rheumatoid-arthritis-patients-treated-with-janus-kinase-inhibitors-and-controls-preliminary-results/