Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Alternative splicing events play a critical role in the immune system1,2 and one of major causal mechanisms for complex traits including immune-mediated diseases (IMDs), but they have been understudied due to the limitation of the short-read sequencing technology3,4. With the emergence of long-read sequencing, large-scale projects have aimed to reconstruct full-length transcripts; however, existing studies focusing on whole blood cells or lymphoblastoid cell lines5,6. The profiling of isoforms by cell types will help elucidate complicated immune system networks and unknown pathogenesis of IMDs.
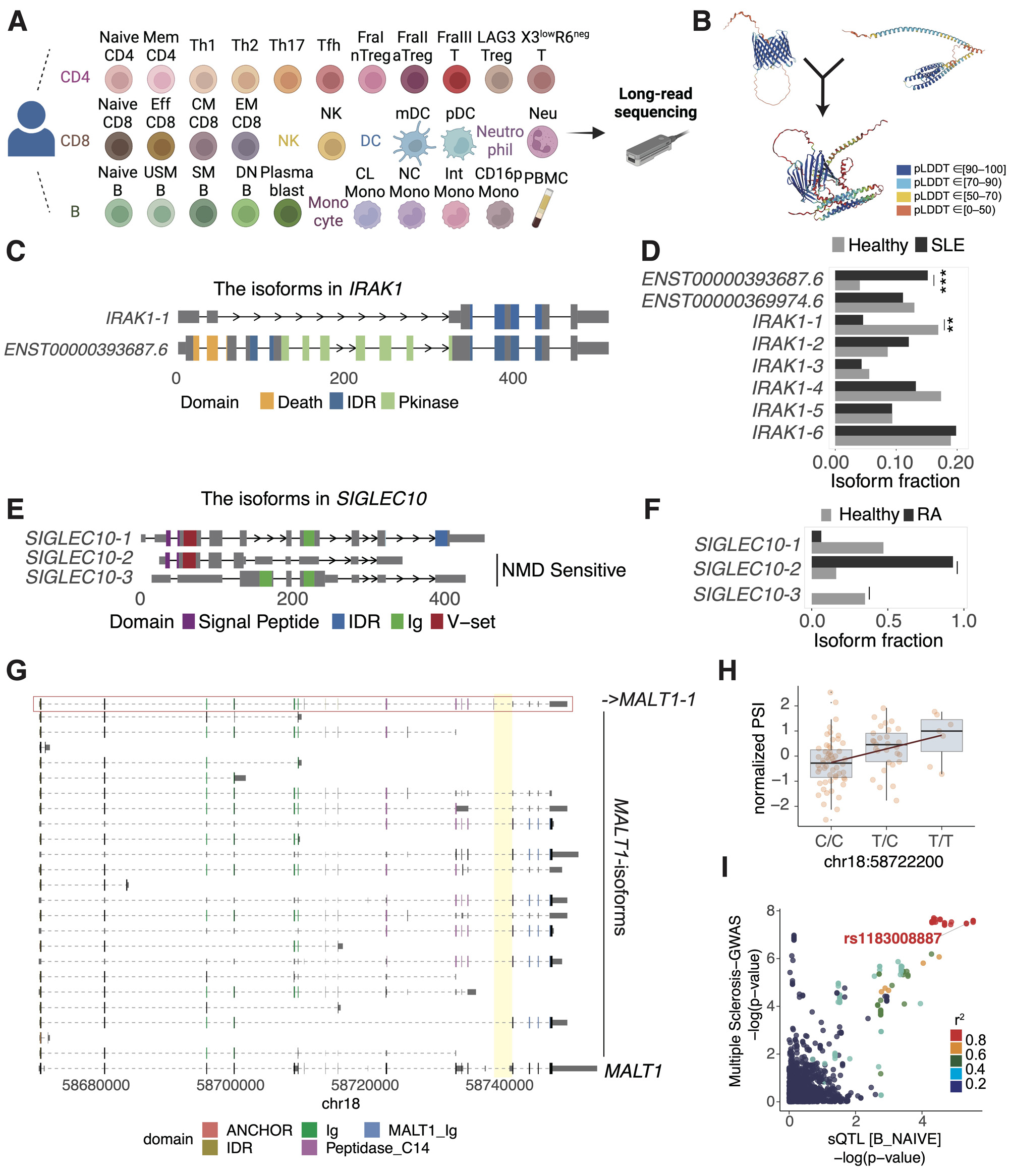
Methods: We isolated 29 immune cell subsets from the peripheral blood cells of a healthy donor (42 years-old female) (Figure 1A). cDNA libraries were made from the poly(A) mRNA, PCR amplified, and subjected to long-read sequencing using the MinION. After mapping raw reads and quality control steps, we generated the atlas containing the full-length of isoforms.
Results: Our atlas (Immune Isoform Atlas) contained a total of 159,369 isoforms transcribed from 17,496 genomic loci. We discovered novel isoforms such as a read-through transcript from the TOMM40-APOE locus, known as the Alzheimer’s disease locus (Figure 1B). Further, we identified disease-associated isoforms by remapping short-read RNA-seq datasets from SLE7, RA8 individuals and relevant controls to the Immune Isoform Atlas (isoform switch analysis). For SLE, we identified 84 genes whose isoform fractions were significantly switched between SLE and healthy individuals (false discovery rate < 0.05). For example, the expression of the known isoform of IRAK1 (ENST 00000393687.6) with a protein kinase domain increased in SLE individuals compared to controls, while the novel IRAK1-1 without this domain decreased (Figure 1C-D). As to RA, we found that one of the novel isoforms in SIGLEC10 being sensitive to nonsense-mediated mRNA decay was more highly expressed in RA compared to controls (Figure 1E-F). Finally, to investigate disease-causal isoforms, we evaluated the colocalization between loci identified by Genome-Wide Association Studies (GWAS) for autoimmune diseases and splicing quantitative trait loci (sQTL) signals using coloc9. Among them, an SNP in MALT1 (rs11873030), which was associated with multiple sclerosis10, had an sQTL effect for a junction read unique to MALT1-1; this isoform decreased with the risk allele for the disease (Figure 1G-I).
Conclusion: Our atlas, obtained by long-read RNA sequencing of 29 immune cell subsets, provided a comprehensive full-length isoform profiling in the human primary immune cells. Our atlas will help reveal unknown pathogenic mechanisms via alternative splicing.
(A) Summary of the cell subsets included in long-read RNA sequencing in our study.
(B) Protein 3D structures of TOMM40 (left), APOE (right), and the read-though isoform (bottom) predicted using AlphaFold2. pLDDT is a per-residue estimate of its confidence on a scale from 0 – 100. Regions with pLDDT > 90, between 70 and 90, between 50 and 70, and < 50 are expected to be high accuracy, well (a generally good backbone prediction), low confidence, and a reasonably strong predictor of disorder, respectively.
(C) Structures of isoforms transcribed from the IRAK1 locus. The x-axis shows distance from the TSS.
(D) Isoform fractions in IRAK1 in SLE and healthy individuals. The significance of comparison is as follows: ***, p<0.001; **, p<0.01; *, p<0.05.
(E) Structures of isoforms transcribed from the SIGLEC10 locus.
(F) Isoform fractions in SIGLEC10 in RA and healthy individuals.
(G) Structures of isoforms transcribed from MALT1. The collapsed gene structure registered in GENCODE is shown at the bottom.
(H) sQTL plot of normalized PSI of the unique junction (chr18:58739121-58741865, highlighted in yellow in Figure 1G) in the related isoform (MALT1_1, highlighted in red in Figure 1G) in naïve B cells.
(I) Colocalization plot of sQTL and GWAS of multiple sclerosis.
To cite this abstract in AMA style:
Inamo J, Suzuki A, Takahashi Ueda M, Yamaguchi K, Nishida H, Suzuki K, Kaneko Y, Takeuchi T, Hatano H, Ishigaki K, Ishihama Y, Yamamoto K, Kochi Y. Immune Isoform Atlas: Landscape of Alternative Splicing in Human Immune Cells and Involvement of Dysregulated Alternative Splicing in Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immune-isoform-atlas-landscape-of-alternative-splicing-in-human-immune-cells-and-involvement-of-dysregulated-alternative-splicing-in-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-isoform-atlas-landscape-of-alternative-splicing-in-human-immune-cells-and-involvement-of-dysregulated-alternative-splicing-in-autoimmune-diseases/